Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**Dosage and Administration** **Pharmaceutical Form** Tablet. **Posology** _First-line ovarian cancer maintenance treatment_ The recommended starting dose of _ZEJULA_ is 200 mg taken once daily. However, for those patients who weigh ≥ 77 kg and have baseline platelet count ≥ 150,000/microlitre, the recommended starting dose of _ZEJULA_ is 300 mg taken once daily. For the maintenance treatment of advanced ovarian cancer, patients should start treatment with _ZEJULA_ no later than 12 weeks after their most recent platinum-containing regimen. _Recurrent ovarian cancer maintenance treatment_ The dose is 300 mg once daily. _Patients with low body weight in recurrent ovarian cancer maintenance treatment_ Approximately 25 % of patients in the NOVA study weighed less than 58 kg, and approximately 25 % of patients weighed more than 77 kg. The incidence of Grade 3 or 4 ADRs was greater among low body weight patients (78 %) than high body weight patients (53 %). Only 13 % of low body weight patients remained at a dose of 300 mg beyond Cycle 3. A starting dose of 200 mg for patients weighing less than 58 kg may be considered. For the maintenance treatment of recurrent ovarian cancer, patients should start treatment with _ZEJULA_ no later than 8 weeks after their most recent platinum-containing regimen. Patients should be encouraged to take their dose at approximately the same time each day. Bedtime administration may be a potential method for managing nausea. Treatment should be continued until disease progression or unacceptable toxicity. _Missing dose_ If patients miss a dose, they should take their next dose at its regularly scheduled time. _Dose adjustments for adverse reactions_ Recommendations for dose modifications for adverse reactions are provided in Tables 1, 2 and 3. In general, it is recommended to first interrupt the treatment (but no longer than 28 consecutive days) to allow the patient to recover from the adverse reaction and then restart at the same dose. In the case that the adverse reaction recurs, it is recommended to interrupt the treatment and then resume at the lower dose. If adverse reactions persist beyond a 28-day dose interruption, it is recommended that _ZEJULA_ be discontinued. If adverse reactions are not manageable with this strategy of dose interruption and reduction, it is recommended that _ZEJULA_ be discontinued. 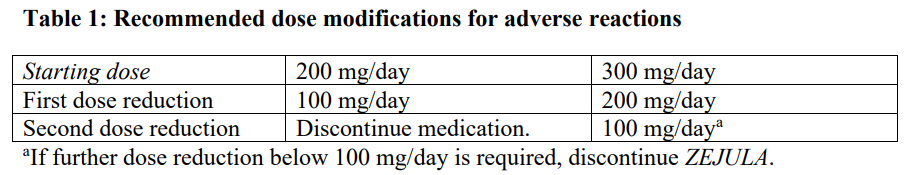 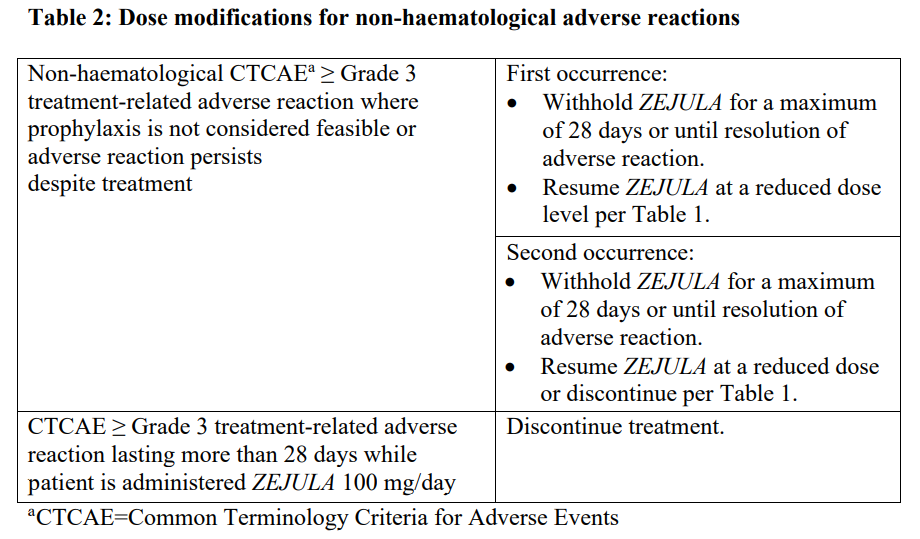 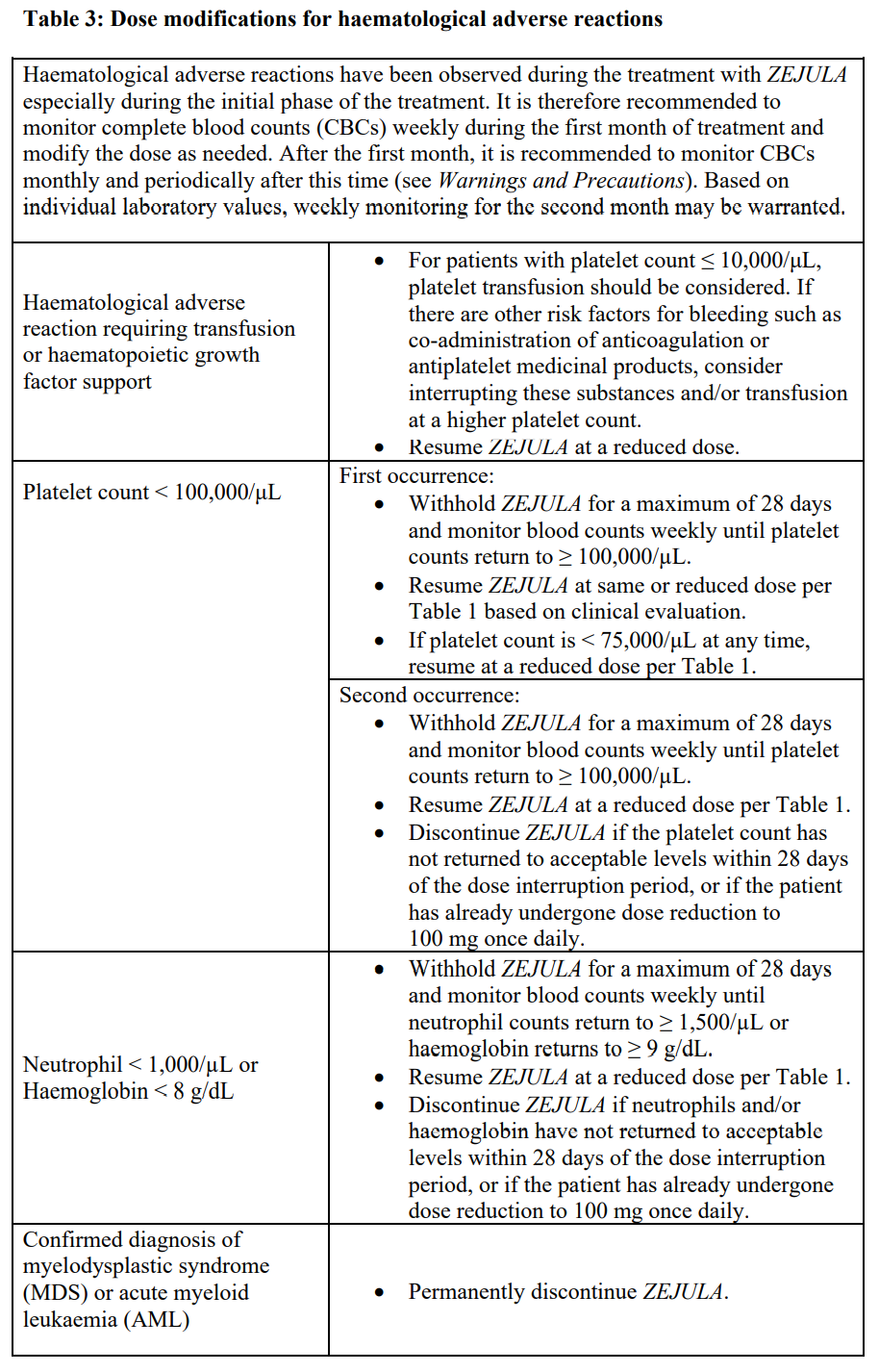 **Method of Administration** Swallow tablets whole with water. Do not chew or crush tablets. _ZEJULA_ can be taken without regard to meals ( _see Pharmacokinetics_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Children and Adolescents** The safety and efficacy of niraparib in children and adolescents below 18 years of age have not yet been established. **Elderly** No dose adjustment is necessary for elderly patients (≥ 65 years). There are limited clinical data in patients aged 75 years or over. **Renal impairment** No dose adjustment is necessary for patients with mild to moderate renal impairment. There are no data in patients with severe renal impairment or end stage renal disease undergoing haemodialysis; use with caution in these patients (see _Pharmacokinetics_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Hepatic impairment** No dose adjustment is needed in patients with mild hepatic impairment (either aspartate aminotransferase (AST) >upper limit of normal (ULN) and total bilirubin (TB) ≤ULN or any AST and TB > 1.0x – 1.5x ULN). For patients with moderate hepatic impairment (any AST and TB > 1.5x – 3x ULN), the recommended starting dose of _ZEJULA_ is 200 mg once daily. There are no data in patients with severe hepatic impairment (any AST and TB > 3x ULN); use with caution in these patients (see _Pharmacokinetics_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
ORAL
Medical Information
**Indications** _ZEJULA_ is indicated: - as monotherapy for the maintenance treatment of adult patients with advanced epithelial (FIGO stages III and IV) high-grade ovarian, fallopian tube or primary peritoneal cancer who are in response (complete or partial) following completion of first-line platinum-based chemotherapy. - as monotherapy for the maintenance treatment of adult patients with platinum-sensitive relapsed high grade serous epithelial ovarian, fallopian tube, or primary peritoneal cancer who are in response (complete or partial) to platinum-based chemotherapy. The overall survival benefit in patients without germline BRCA mutation ovarian cancer has not been demonstrated (see _Clinical Studies_ section – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
**Contraindications** Hypersensitivity to the niraparib or to any of the excipients listed in Excipients – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. Breast-feeding (see _Pregnancy and Lactation_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
L01XK02
niraparib
Manufacturer Information
GLAXOSMITHKLINE PTE LTD
Mayne Pharma Inc.
Millmount Healthcare Ltd (Primary & Secondary Packager)
