Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FOR SUSPENSION
**Dosage and Administration** Pharmaceutical form: Dispersible tablets. **Posology** _TIVICAY_ therapy should be initiated by a physician experienced in the management of HIV infection. _TIVICAY_ is available as dispersible tablets for patients aged at least 4 weeks and weighing at least 3 kg, or for patients in whom film-coated tablets are not appropriate. _TIVICAY_ is available as film-coated tablets for patients aged at least 6 years and weighing at least 14 kg. The bioavailability of dispersible tablets and film-coated tablets is not comparable therefore they must not be used as direct replacements ( _see Pharmacokinetics_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). For example, the recommended adult dose for dispersible tablets is 30 mg versus 50 mg for film-coated tablets. Patients changing between dispersible and film-coated tablets should follow the dosing recommendations that are specific for the formulation. _TIVICAY_ can be taken with or without food. The dispersible tablets may be swallowed whole with drinking water or dispersed in drinking water. When dispersed, the amount of water will depend on the number of tablets prescribed. The tablet(s) should be fully dispersed before swallowing ( _see Instructions for Use_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Do not chew, cut or crush the tablets. **Method of Administration** **Adults** **Patients infected with HIV-1 without resistance to the integrase class** The recommended dose of dolutegravir dispersible tablets is 30 mg once daily. **Patients infected with HIV-1 with resistance to the integrase class (documented or clinically suspected)** The recommended dose of dolutegravir dispersible tablets is 30 mg twice daily. The decision to use dolutegravir for such patients should be informed by the integrase resistance pattern ( _see Clinical studies_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Adolescents, children and infants aged at least 4 weeks and weighing at least 3 kg** **Patients infected with HIV-1 without resistance to the integrase class** The recommended dose of dolutegravir dispersible tablets is determined according to weight and age and is presented in the table below. 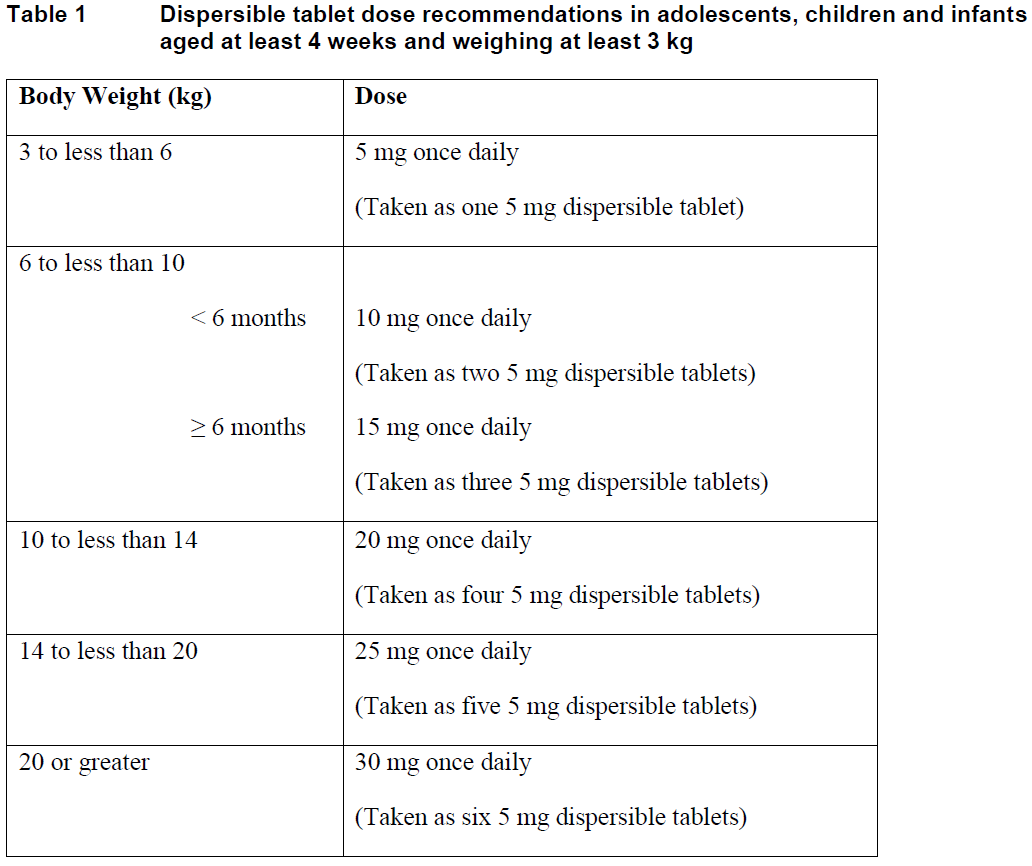 If swallowing the dispersible tablets whole with water, do not swallow more than one tablet at a time to reduce the risk of choking. There are insufficient safety and efficacy data available to recommend a dose for dolutegravir dispersible tablets in children below age 4 weeks or weighing less than 3 kg. **Patients infected with HIV-1 with resistance to the integrase class** There are insufficient data to recommend a dose for dolutegravir dispersible tablets in integrase inhibitor resistant adolescents, children and infants. **Missed doses** If the patient misses a dose of _TIVICAY_, the patient should take _TIVICAY_ as soon as possible, providing the next dose is not due within 4 hours. If the next dose is due within 4 hours, the patient should not take the missed dose and simply resume the usual dosing schedule. **Elderly** There are limited data available on the use of _TIVICAY_ in patients aged 65 years and over. However, there is no evidence that elderly patients require a different dose than younger adult patients ( _see Pharmacokinetics – Special Patient Populations_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Renal impairment** No dosage adjustment is required in patients with mild, moderate or severe (creatinine clearance (CrCl) <30 mL /min, not on dialysis) renal impairment. Limited data are available in subjects receiving dialysis, although differences in pharmacokinetics are not expected in this population ( _see Pharmacokinetics – Special Patient Populations_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Hepatic impairment** No dosage adjustment is required in patients with mild or moderate hepatic impairment (Child-Pugh grade A or B). No data are available in patients with severe hepatic impairment (Child-Pugh grade C) ( _see Pharmacokinetics – Special Patient Populations_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
ORAL
Medical Information
**Indications** Treatment of human immunodeficiency virus (HIV) infection in combination with other antiretroviral agents in adults and children aged at least 4 weeks of age or older and weighing at least 3 kg.
**Contraindications** _TIVICAY_ must not be administered concurrently with medicinal products with narrow therapeutic windows, that are substrates of organic cation transporter 2 (OCT2), including but not limited to dofetilide, pilsicainide or fampridine (also known as dalfampridine; _see Interactions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _TIVICAY_ is contraindicated in patients with known hypersensitivity to dolutegravir or to any of the excipients.
J05AX12
xj 05 ax 12
Manufacturer Information
GLAXOSMITHKLINE PTE LTD
Glaxo Operations UK Ltd (trading as Glaxo Wellcome Operations)
Glaxo Wellcome, S.A. (Primary packager and Secondary packager)
