Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
SOLUTION
**DOSAGE AND ADMINISTRATION** Apply Loceryl Nail Lacquer to the affected nails at a dosage of 1 to 2 applications weekly. When applying the varnish carefully comply with the following recommendations. 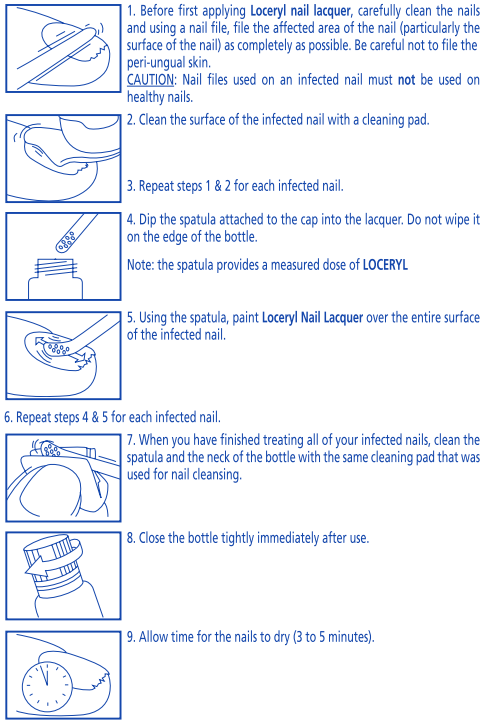 **NOTE**: Follow these steps each time you use **LOCERYL.** When working with organic solvents (thinners, white spirit, etc) wear impermeable gloves in order to protect the **LOCERYL** lacquer on the nails. Treatment should be continued without interruption until the nail is regenerated and the affected areas are finally cured. The required duration of treatment depends essentially on intensity and the localization of the infection and on the growth rate of nails. In general, it is 6 months for fingernails and 9 to 12 months for toe nails. If there is no improvement, or only small improvement after 6 to 12 months of treatment, see your healthcare professional
TOPICAL
Medical Information
**INDICATIONS AND USAGE** **Loceryl Nail Lacquer** is indicated in the topical treatment of nail fungal infections (onychomycosis) caused by dermatophytes, yeasts and moulds.
**CONTRA-INDICATIONS** **Loceryl Nail Lacquer** must not be reused by patients who have shown hypersensitivity to any ingredient of the product.
D01AE16
amorolfine
Manufacturer Information
GALDERMA SINGAPORE PRIVATE LIMITED
LABORATOIRES GALDERMA
Active Ingredients
Documents
Patient Information Leaflets
Loceryl Nail Lacquer 5% PIL.pdf
Approved: December 3, 2018
