Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, SOLUTION
**DOSAGE AND ADMINISTRATION** The recommended dosage regimen of ALDURAZYME is 0.58 mg/kg of body weight administered once-weekly as an intravenous (IV) infusion. Pretreatment is recommended 60 minutes prior to the start of the infusion and may include antihistamines, antipyretics or both (see section Warnings and Precautions – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Each vial of ALDURAZYME provides 2.9 milligrams (mg) of laronidase in 5.0 milliliters (mL) of solution and is intended for single use only. Do not use the vial more than one time. The concentrated solution for infusion must be diluted with 0.9% Sodium Chloride Injection, USP, to a final volume of 100 mL or 250 mL, using aseptic techniques. The final volume of the infusion is determined by the patient’s body weight. Patients with a body weight of 20 kg or less should receive a total volume of 100 m L. Patients with a body weight greater than 20 kg should receive a total volume of 250 mL (see section Dosage and Administration). For patients with underlying cardiac or respiratory compromise and weighing up to 30 kg, physicians may consider diluting ALDURAZYME in a volume of 100 mL and administering at a decreased infusion rate ( _see Dosage and Administration, Warnings and Precautions and Adverse Reactions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Instructions for Use** Prepare and use ALDURAZYME according to the following steps. Use aseptic techniques. Prepare ALDURAZYME using low-protein-binding containers and administer with a low-protein-binding infusion set equipped with an in-line, low-protein-binding 0.2 micron filter. There is no information on the compatibility of diluted ALDURAZYME with glass containers. 1. Determine the number of vials to be diluted based on the patient’s weight and the recommended dose of 0.58 mg/kg, using the following equation: Patient’s weight (kg) × 1 mL/kg of ALDURAZYME = Total number of mL of ALDURAZYME Total number of mL of ALDURAZYME ÷ 5 mL per Vial = Total number of Vials. 2. Round up to the next whole vial. Remove the required number of vials from the refrigerator to allow them to reach room temperature. Do not heat or microwave vials. 3. Before withdrawing the ALDURAZYME from the vial, visually inspect each vial for particulate matter and discoloration. The ALDURAZYME solution should be clear to slightly opalescent and colorless to pale yellow. Some translucency may be present in the solution. Do not use if the solution is discolored or if there is particulate matter in the solution. 4. Withdraw and discard a volume of the 0.9% Sodium Chloride Injection, USP from the infusion bag, equal to the volume of ALDURAZYME concentrate to be added. 5. Slowly withdraw the calculated volume of ALDURAZYME from the appropriate number of vials using caution to avoid excessive agitation. Do not use a filter needle, as this may cause agitation. Agitation may denature ALDURAZYME, rendering it biologically inactive. 6. Slowly add the ALDURAZYME solution to the 0.9% Sodium Chloride Injection, USP using care to avoid agitation of the solutions. Do not use a filter needle. 7. Gently rotate the infusion bag to ensure proper distribution of ALDURAZYME. Do not shake the solution. 8. The entire infusion volume (100 mL for patients weighing 20 kg or less and 250 mL for patients weighing greater than 20 kg) should be delivered over approximately 3 to 4 hours. The initial infusion rate of 10 mcg/kg/hr may be incrementally increased every 15 minutes during the first hour, as tolerated, until a maximum infusion rate of 200 mcg/kg/hr is reached. The maximum rate is then maintained for the remainder of the infusion (2–3 hours), as outlined in _Tables 1 and 2_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. 9. Administer the diluted ALDURAZYME solution to patients using a low-protein-binding infusion set equipped with a low-protein-binding 0.2 micrometres in-line filter. 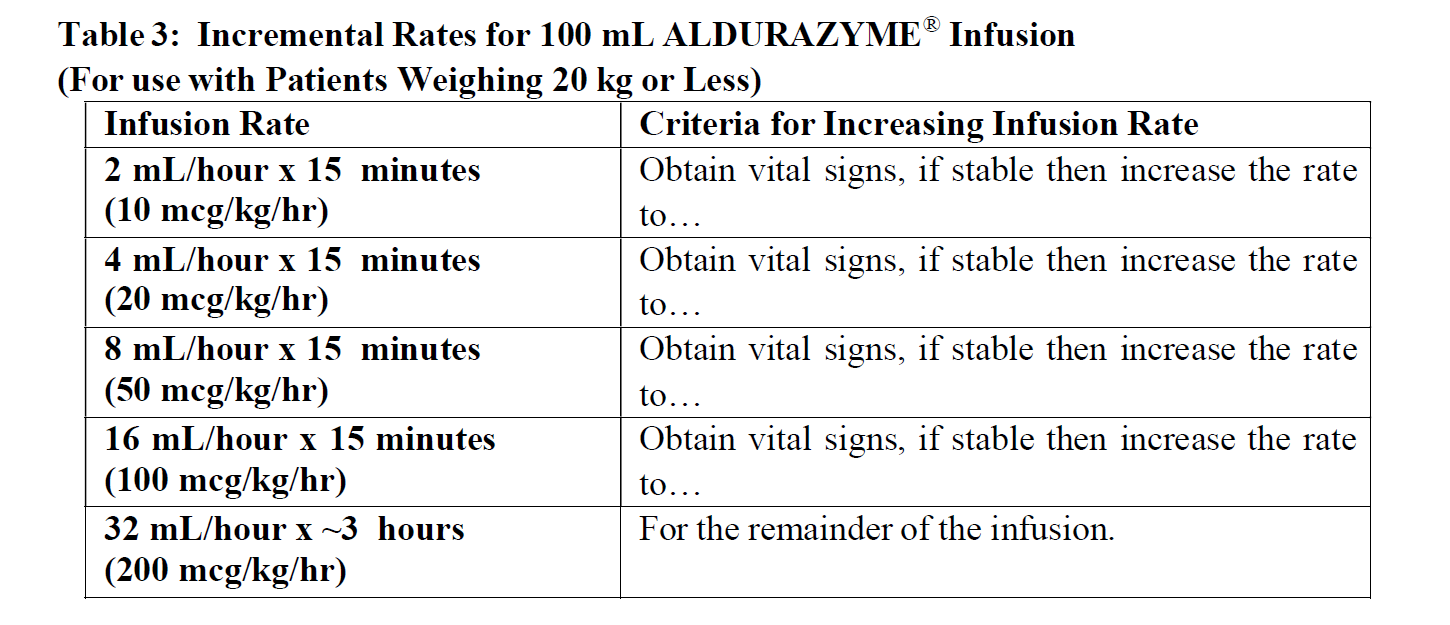 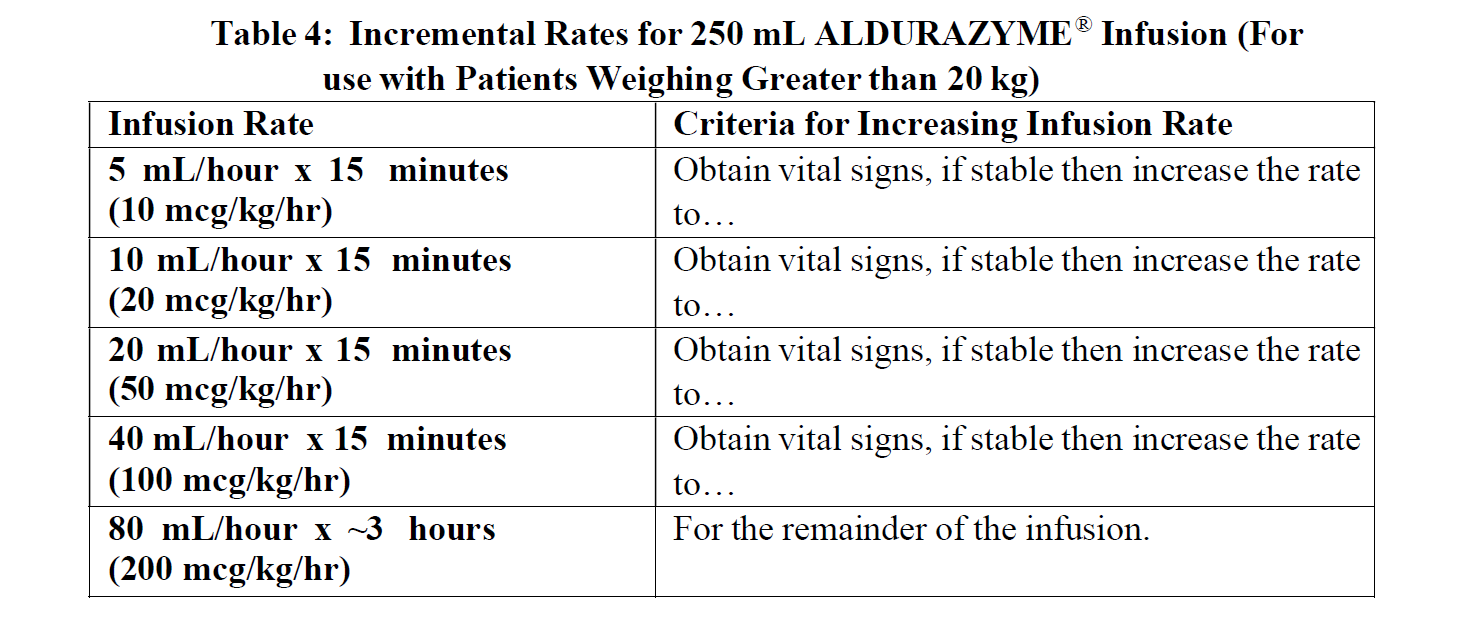 ALDURAZYME does not contain any preservatives, therefore, after dilution with saline, the infusion bags should be used immediately. If immediate use is not possible, the diluted solution should be stored refrigerated at 2°C to 8°C (36°F to 46°F) for up to 36 hours. Other than during infusion, room temperature storage of diluted solution is not recommended. Any unused product or waste material should be discarded and disposed of in accordance with local requirements. ALDURAZYME must not be administered with other medicinal products in the same infusion. The compatibility of ALDURAZYME in solution with other products has not been evaluated.
INTRAVENOUS
Medical Information
**INDICATIONS AND USAGE** ALDURAZYME (laronidase) is indicated for patients with Hurler and Hurler-Scheie forms of Mucopolysaccharidosis I (MPS I) and for patients with the Scheie form who have moderate to severe symptoms. The risks and benefits of treating mildly affected patients with the Scheie form have not been established. - ALDURAZYME has been shown to improve pulmonary function and walking capacity. ALDURAZYME has not been evaluated for effects on the central nervous system manifestations of the disorder.
**CONTRAINDICATIONS** None.
A16AB05
laronidase
Manufacturer Information
SANOFI-AVENTIS SINGAPORE PTE. LTD.
Vetter Pharma-Fertigung GmbH & Co. KG
BIOMARINE PHARMACEUTICAL INC
Active Ingredients
Documents
Package Inserts
Aldurazyme PI.pdf
Approved: June 18, 2021
