Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**DOSAGE AND ADMINISTRATION** **Transfusional iron overload** **Dosage** It is recommended that therapy with Jadenu be started after the transfusion of approximately 20 units (about 100 mL/kg) of packed red blood cells or when there is evidence from clinical monitoring that chronic iron overload is present (e.g. serum ferritin >1,000 microgram/L). Doses (in mg/kg) must be calculated and rounded to the nearest whole tablet size. The goals of iron chelation therapy are to remove the amount of iron administered in transfusions and, as required, to reduce the existing iron burden. The decision to remove accumulated iron should be individualized based on anticipated clinical benefit and risks of chelation therapy. Jadenu film-coated tablets are a strength-adjusted formulation of deferasirox with higher bioavailability compared to the Exjade dispersible tablet formulation (see section CLINICAL PHARMACOLOGY – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). For patients who are currently on chelation therapy with Exjade dispersible tablet and switching to Jadenu, the dose of Jadenu should be 30% lower than the dose of Exjade, rounded to the nearest whole tablet, as shown in Table 3. **Starting dose** The recommended initial daily dose of Jadenu is 14 mg/kg body weight. An initial daily dose of 21 mg/kg may be considered for patients receiving more than 14 mL/kg/month of packed red blood cells (approximately >4 units/month for an adult), and for whom the objective is reduction of iron overload. An initial daily dose of 7 mg/kg may be considered for patients receiving less than 7 mL/kg/month of packed red blood cells (approximately <2 units/month for an adult), and for whom the objective is maintenance of the body iron level. For patients already well-managed on treatment with deferoxamine, a starting dose of Jadenu that is numerically one third of the deferoxamine dose could be considered as shown in Table 1 and 3 (e.g. a patient receiving 40 mg/kg/day of deferoxamine for 5 days per week (or equivalent) could be transferred to a starting daily dose of 14 mg/kg/day of Jadenu). **Dose adjustment** It is recommended that serum ferritin be monitored every month and that the dose of Jadenu is adjusted if necessary every 3 to 6 months based on the trends in serum ferritin. Dose adjustments may be made in steps of 3.5 to 7 mg/kg and are to be tailored to the individual patient’s response and therapeutic goals (maintenance or reduction of iron burden). In patients not adequately controlled with doses of 21 mg/kg (e.g. serum ferritin levels persistently above 2500 microgram/L and not showing a decreasing trend over time), doses of up to 28 mg/kg may be considered. Doses above 28 mg/kg are not recommended because there is only limited experience with doses above this level. In patients whose serum ferritin level has reached the target (usually between 500 and 1,000 microgram/L), dose reductions in steps of 3.5 to 7 mg/kg should be considered to maintain serum ferritin levels within the target range and to minimize the risk of overchelation (see section WARNINGS AND PRECAUTIONS – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). If serum ferritin falls consistently below 500 microgram/L, an interruption of treatment should be considered. As with other iron chelator treatment, the risk of toxicity of Jadenu may be increased when inappropriately high doses are given in patients with a low iron burden or with serum ferritin levels that are only slightly elevated (see section WARNINGS AND PRECAUTIONS – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The corresponding recommended doses for both formulations are shown in Table 1. 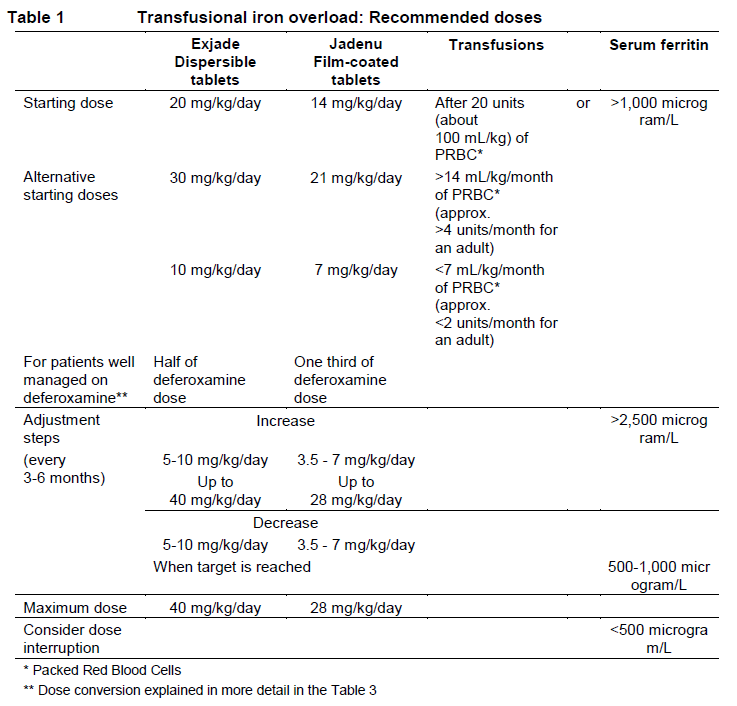 **Non-transfusion-dependent thalassemia (NTDT) syndromes** **Dosage** Chelation therapy should only be initiated when there is evidence of iron overload (liver iron concentration (LIC) ≥5 mg Fe/g dry weight (dw) or serum ferritin consistently >800 microgram/L). In patients with no LIC assessment, caution should be taken during chelation therapy to minimize the risk of overchelation. Jadenu film-coated tablets are a strength-adjusted formulation of deferasirox with higher bioavailability compared to the Exjade dispersible tablet formulation (see section CLINICAL PHARMACOLOGY – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). For patients who are currently on chelation therapy with Exjade dispersible tablet and switching to Jadenu, the dose of Jadenu should be 30% lower than the dose of Exjade, rounded to the nearest whole tablet. **Starting dose** The recommended initial daily dose of Jadenu is 7 mg/kg body weight. **Dose adjustment** It is recommended that serum ferritin be monitored every month to assess the patient's response to therapy and to minimize the risk of overchelation (see section WARNINGS AND PRECAUTIONS – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Every 3 to 6 months of treatment, consider a dose increase in increments of 3.5 to 7 mg/kg if the patient’s LIC is ≥7 mg Fe/g dw, or serum ferritin is consistently >2,000 microgram/L and not showing a downward trend, and the patient is tolerating the drug well. Doses above 14 mg/kg are not recommended because there is no experience with doses above this level in patients with non-transfusion-dependent thalassemia syndromes. In patients in whom LIC was not assessed and serum ferritin is ≤2,000 microgram/L, dosing should not exceed 7 mg/kg. For patients in whom the dose was increased to >7 mg/kg, dose reduction is recommended to 7 mg/kg or less when LIC is <7 mg Fe/g dw or serum ferritin is ≤2,000 microgram/L. Once a satisfactory body iron level has been achieved (LIC <3 mg Fe/g dw or serum ferritin <300 microgram/L), treatment should be interrupted. Treatment should be re-initiated when there is evidence from clinical monitoring that chronic iron overload is present. The corresponding recommended doses for both formulations are shown in Table 2. 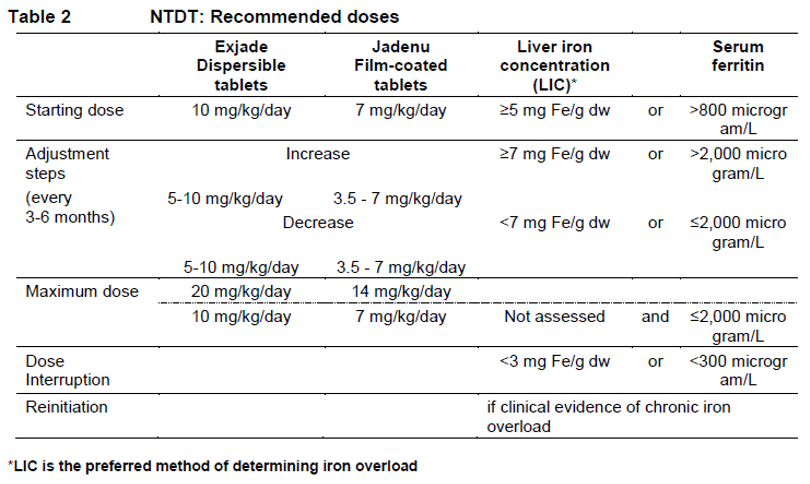 **Transfusional iron overload and non-transfusion-dependent thalassemia syndromes** Information on dose conversion between Exjade dispersible tablet and Jadenu film-coated tablet, as well as deferoxamine is shown in Table 3 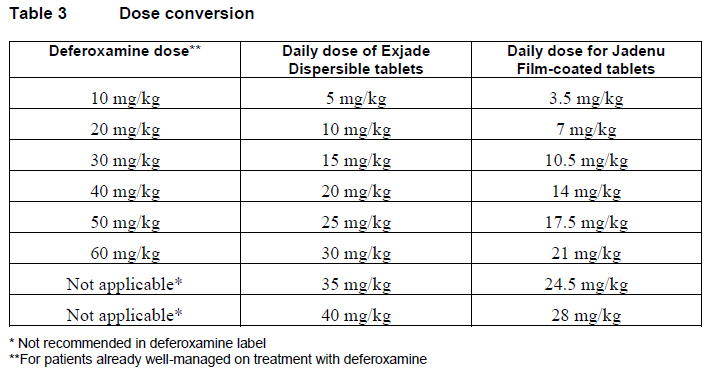 **Special populations** **Patients with renal impairment** Jadenu has not been studied in patients with renal impairment and is contraindicated in patients with estimated creatinine clearance <60 ml/min (see sections CONTRAINDICATIONS and WARNINGS AND PRECAUTIONS – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Jadenu treatment must be used with caution in patients with serum creatinine levels above the age-appropriate upper limit of the normal range. The initial dosing recommendations for patients with renal impairment are the same as described above. Serum creatinine should be monitored monthly in all patients and if necessary daily doses can be reduced by 7 mg/kg (see section WARNINGS AND PRECAUTIONS – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Patients with hepatic impairment** Deferasirox has been studied in a clinical trial in subjects with hepatic impairment. For patients with moderate hepatic impairment (Child-Pugh B), the starting dose should be reduced by approximately 50%. Jadenu should not be used in patients with severe hepatic impairment (Child-Pugh C) (see section WARNINGS AND PRECAUTIONS and section CLINICAL PHARMACOLOGY – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Hepatic function in all patients should be monitored before the initiation of treatment, every 2 weeks during the first month and monthly thereafter (see section WARNINGS AND PRECAUTIONS – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Pediatric patients** The dosing recommendations for pediatric patients are the same as for adult patients. It is recommended that serum ferritin be monitored every month to assess the patient's response to therapy and to minimize the risk of overchelation (see section WARNINGS AND PRECAUTIONS – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Changes in weight of pediatric patients over time must be taken into account when calculating the dose. **Elderly patients** The dosing recommendations for elderly patients are the same as described above. In clinical trials, elderly patients experienced a higher frequency of adverse reactions than younger patients and should be monitored closely for adverse reactions that may require a dose adjustment. **Method of administration** The film-coated tablets should be swallowed whole with some water. For patients who are unable to swallow whole tablets, Jadenu film-coated tablets may be crushed and administered by sprinkling the full dose on soft food like yogurt or apple sauce (apple puree). The dose should be immediately and completely consumed, and not stored for future use. Jadenu should be taken once a day, preferably at the same time each day, and may be taken on an empty stomach or with a light meal (see section CLINICAL PHARMACOLOGY – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
ORAL
Medical Information
**INDICATIONS** Jadenu is indicated for the treatment of chronic iron overload due to frequent blood transfusions (≥7 ml/kg/month of packed red blood cells) in patients with beta thalassaemia major aged 6 years and older. Jadenu is also indicated for the treatment of chronic iron overload due to blood transfusions when deferoxamine therapy is contraindicated or inadequate in the following patient groups: - in patients with other anaemias, - in patients aged 2 to 5 years, - in patients with beta thalassaemia major with iron overload due to infrequent blood transfusions (<7 ml/kg/month of packed red blood cells). Jadenu is also indicated for the treatment of chronic iron overload in patients with non-transfusion-dependent thalassemia syndromes aged 10 years and older.
**CONTRAINDICATIONS** Creatinine clearance <60 mL/min or serum creatinine >2 times the age-appropriate upper limit of normal. High-risk myelodysplastic syndrome (MDS) patients and patients with other hematological and non-hematological malignancies who are not expected to benefit from chelation therapy due to the rapid progression of their disease. Hypersensitivity to the active substance or to any of the excipients.
V03AC03
deferasirox
Manufacturer Information
NOVARTIS (SINGAPORE) PTE LTD
Novartis Pharma Stein AG
Novartis Pharma Produktions GmbH (primary and secondary packager)
SANDOZ S.R.L
Active Ingredients
Documents
Package Inserts
Jadenu Tablet PI.pdf
Approved: March 17, 2022
