Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, SOLUTION
**2 DOSAGE AND ADMINISTRATION** **2.1 Important Dosage and Administration Information** Discontinue oral phosphate and/or active vitamin D analogs (e.g. calcitriol, paricalcitol, doxercalciferol, calcifediol) 1 week prior to initiation of treatment _\[see Contraindications (4)_\]. Fasting serum phosphorus concentration should be below the reference range for age prior to initiation of treatment _\[see Contraindications (4)\]_. CRYSVITA is administered by subcutaneous injection and should be administered by a healthcare provider. The maximum volume of CRYSVITA per injection is 1.5 mL. If multiple injections are required, administer at different injection sites. **2.2 Pediatric Patients with X-linked Hypophosphatemia (1 to less than 18 years of age)** The recommended starting dose regimen is 0.8 mg/kg of body weight, rounded to the nearest 10 mg, administered every two weeks. The minimum starting dose is 10 mg up to a maximum dose of 90 mg. After initiation of treatment with CRYSVITA, measure fasting serum phosphorus every 4 weeks for the first 3 months of treatment, and thereafter as appropriate. If serum phosphorus is above the lower limit of the reference range for age and below 5 mg/dL, continue treatment with the same dose. Follow dose adjustment schedule below to maintain serum phosphorus within the reference range for age. _Dose Adjustment_ Reassess fasting serum phosphorus level 4 weeks after dose adjustment. Do not adjust CRYSVITA more frequently than every 4 weeks. _Dose Increase_: If serum phosphorus is below the reference range for age, the dose may be increased stepwise up to approximately 2 mg/kg, administered every two weeks (maximum dose of 90 mg) according to the dosing schedule shown in Table 1. 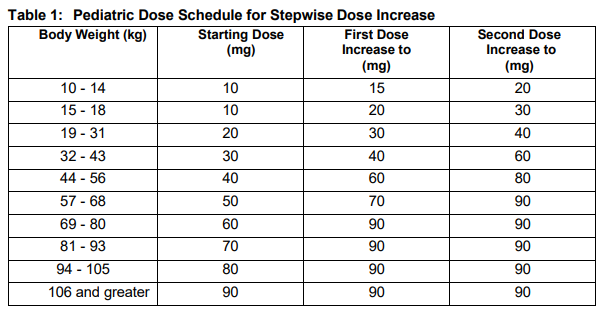 _Dose Decrease:_ If serum phosphorus is above 5 mg/dL, withhold the next dose and reassess the serum phosphorus level in 4 weeks. The patient must have serum phosphorus below the reference range for age to reinitiate CRYSVITA. Once serum phosphorus is below the reference range for age, treatment may be restarted according to the dose schedule shown in Table 2. Reassess serum phosphorus level 4 weeks after dose adjustment. If the level remains below the reference range for age after the re-initiation dose, the dose can be adjusted according to Table 1. 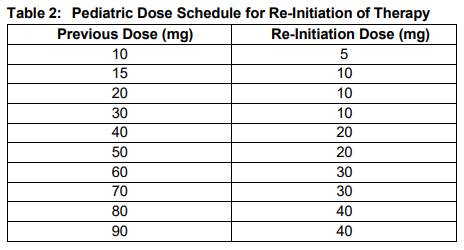 **2.3 Adult Patients with X-linked Hypophosphatemia (18 years of age and older)** The recommended dose regimen in adults is 1 mg/kg body weight, rounded to the nearest 10 mg up to a maximum dose of 90 mg, administered every four weeks. After initiation of treatment with CRYSVITA, assess fasting serum phosphorus on a monthly basis, measured 2 weeks post-dose, for the first 3 months of treatment, and thereafter as appropriate. If serum phosphorus is within the normal range, continue with the same dose. _Dose Decrease:_ Reassess fasting serum phosphorus level 2 weeks after dose adjustment. Do not adjust CRYSVITA more frequently than every 4 weeks. If serum phosphorus is above the normal range, withhold the next dose and reassess the serum phosphorus level after 4 weeks. The patient must have serum phosphorus below the normal range to be able to reinitiate CRYSVITA. Once serum phosphorus is below the normal range, treatment may be restarted at approximately half the initial starting dose up to a maximum dose of 40 mg every 4 weeks according to the dose schedule shown in Table 3. Reassess serum phosphorus 2 weeks after any change in dose. 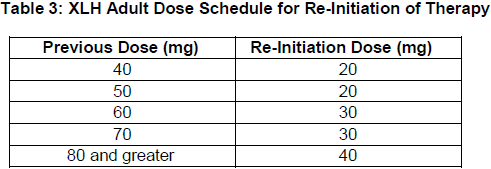 **2.4 Missed Dose** If a patient misses a dose, resume CRYSVITA as soon as possible at the prescribed dose. To avoid missed doses, treatments may be administered 3 days either side of the scheduled treatment date. **2.5 25-Hydroxy Vitamin D Supplementation** Monitor 25-hydroxy vitamin D levels. Supplement with cholecalciferol or ergocalciferol to maintain 25-hydroxy vitamin D levels in the normal range for age. Do not administer active Vitamin D analogs during CRYSVITA treatment _\[see Contraindications (4)\]._ **2.6 General Considerations for Subcutaneous Administration** Injection sites should be rotated with each injection administered at a different anatomic location (upper arms, upper thighs, buttocks, or any quadrant of abdomen) than the previous injection. Do not inject into moles, scars, or areas where the skin is tender, bruised, red, hard, or not intact. If a given dose on a dosing day requires multiple vials of CRYSVITA, contents from two vials can be combined for injection. The maximum volume of CRYSVITA per injection is 1.5 mL. If multiple injections are required on a given dosing day, administer at different injection sites. Monitor for signs of reactions _\[see Warnings and Precautions (5.3)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. Visually inspect CRYSVITA for particulate matter and discoloration prior to administration. CRYSVITA is a sterile, preservative-free, clear to slightly opalescent and colorless to pale brown-yellow solution for subcutaneous injection. Do not use if the solution is discolored or cloudy or if the solution contains any particles or foreign particulate matter.
SUBCUTANEOUS
Medical Information
**1 INDICATIONS AND USAGE** **1.1 X-linked Hypophosphatemia** CRYSVITA is indicated for the treatment of X-linked hypophosphatemia (XLH) in adult and pediatric patients 1 year of age and older.
**4 CONTRAINDICATIONS** CRYSVITA is contraindicated: - In concomitant use with oral phosphate and/or active vitamin D analogs (e.g. calcitriol, paricalcitol, doxercalciferol, calcifediol) due to the risk of hyperphosphatemia _\[see Warnings and Precautions (5.2) and Drug Interactions (7.1)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. - When serum phosphorus is within or above the normal range for age _\[see Warnings and Precautions (5.2)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. - In patients with severe renal impairment or end stage renal disease because these conditions are associated with abnormal mineral metabolism _\[see Use In Specific Population (8.5)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_.
M05BX05
burosumab
Manufacturer Information
KYOWA KIRIN ASIA PACIFIC PTE. LTD.
KYOWA KIRIN CO., LTD., TAKASAKI PLANT
Active Ingredients
Documents
Package Inserts
Rply5IR.Q1_1.4.3Crysvita SG PI_ver.20210114_SC20210115clean.pdf
Approved: May 3, 2021
