Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**2 DOSAGE AND ADMINISTRATION** **2.1 Premedication for Diarrhea** When not using dose escalation _\[see Dosage and Administration (2.2)\]_, administer antidiarrheal prophylaxis during the first 56 days of treatment and initiate with the first dose of NERLYNX \[ _see Warnings and Precautions (5.1) and Adverse Reactions (6.1)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_\]. Instruct patients to take loperamide as directed in Table 1. Titrate Loperamide to 1–2 bowel movements per day. 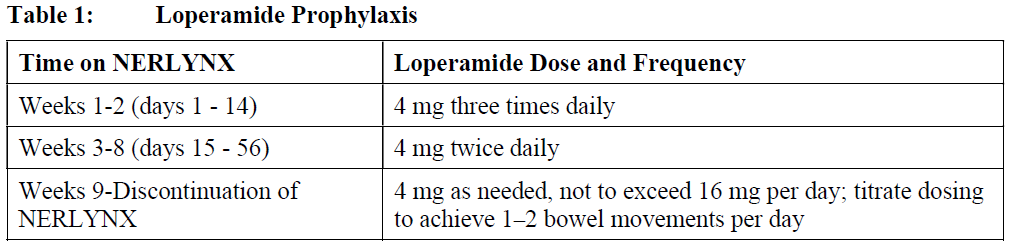 If diarrhea occurs despite prophylaxis, treat with additional antidiarrheals, fluids and electrolytes as clinically indicated. NERLYNX dose interruptions and dose reductions may also be required to manage diarrhea \[ _see Dosage and Administration (2.3)_\]. **2.2 Recommended Dose and Schedule** _**Extended Adjuvant Treatment of Early-Stage Breast Cancer**_ The recommended dose of NERLYNX is 240 mg (six tablets) given orally once daily, with food, continuously until disease recurrence or for up to one year. Treatment with NERLYNX should be initiated within 1 year after completion of trastuzumab therapy. _**Advanced or Metastatic Breast Cancer**_ The recommended dose of NERLYNX is 240 mg (six tablets) given orally once daily with food on Days 1–21 of a 21-day cycle plus capecitabine (750 mg/m2 given orally twice daily) on Days 1–14 of a 21-day cycle until disease progression or unacceptable toxicities. Dose Escalation A two week dose escalation for NERLYNX may be considered instead of starting at the 240 mg daily dose for patients with early-stage breast cancer and metastatic breast cancer, as described in Table 2 _\[see Warnings and Precautions (5.1) and Adverse Reactions (6.1)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. 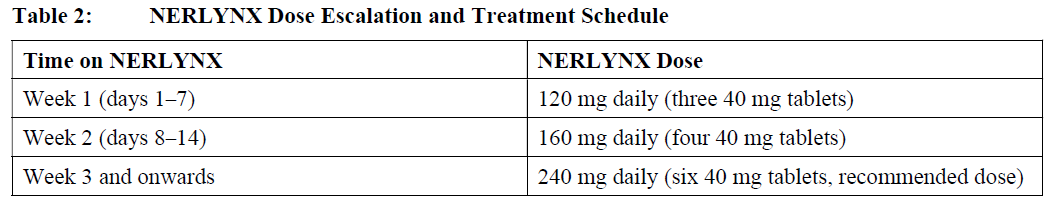 If diarrhea occurs, treat with antidiarrheal medications, fluids, and electrolytes as clinically indicated. NERLYNX dose interruptions and dose reductions may also be required to manage diarrhea _\[see Dosage and Administration (2.3)\]_. Administration Instructions Instruct patients to take NERLYNX at approximately the same time every day. NERLYNX tablets should be swallowed whole (tablets should not be chewed, crushed, or split prior to swallowing). If a patient misses a dose, do not replace missed dose, and instruct the patient to resume NERLYNX with the next scheduled daily dose. **2.3 Dosage Modifications for Adverse Reactions** NERLYNX dose modification is recommended based on individual safety and tolerability. Management of some adverse reactions may require dose interruption and/or dose reduction as shown in Table 3 to Table 6. Discontinue NERLYNX for patients with adverse reactions that fail to recover to Grade 0–1 or baseline, with toxicities that result in a treatment delay > 3 weeks, or if unable to tolerate 120 mg daily. Additional clinical situations may result in dose adjustments as clinically indicated (e.g. intolerable toxicities, persistent Grade 2 adverse reactions, etc.). When NERLYNX is used in combination with capecitabine, refer to the capecitabine prescribing information for dose modifications of capecitabine. 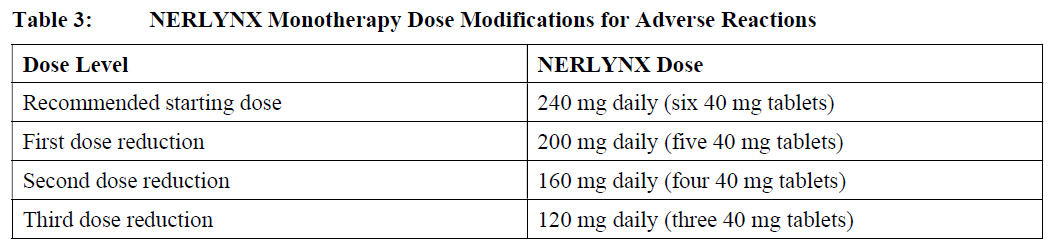 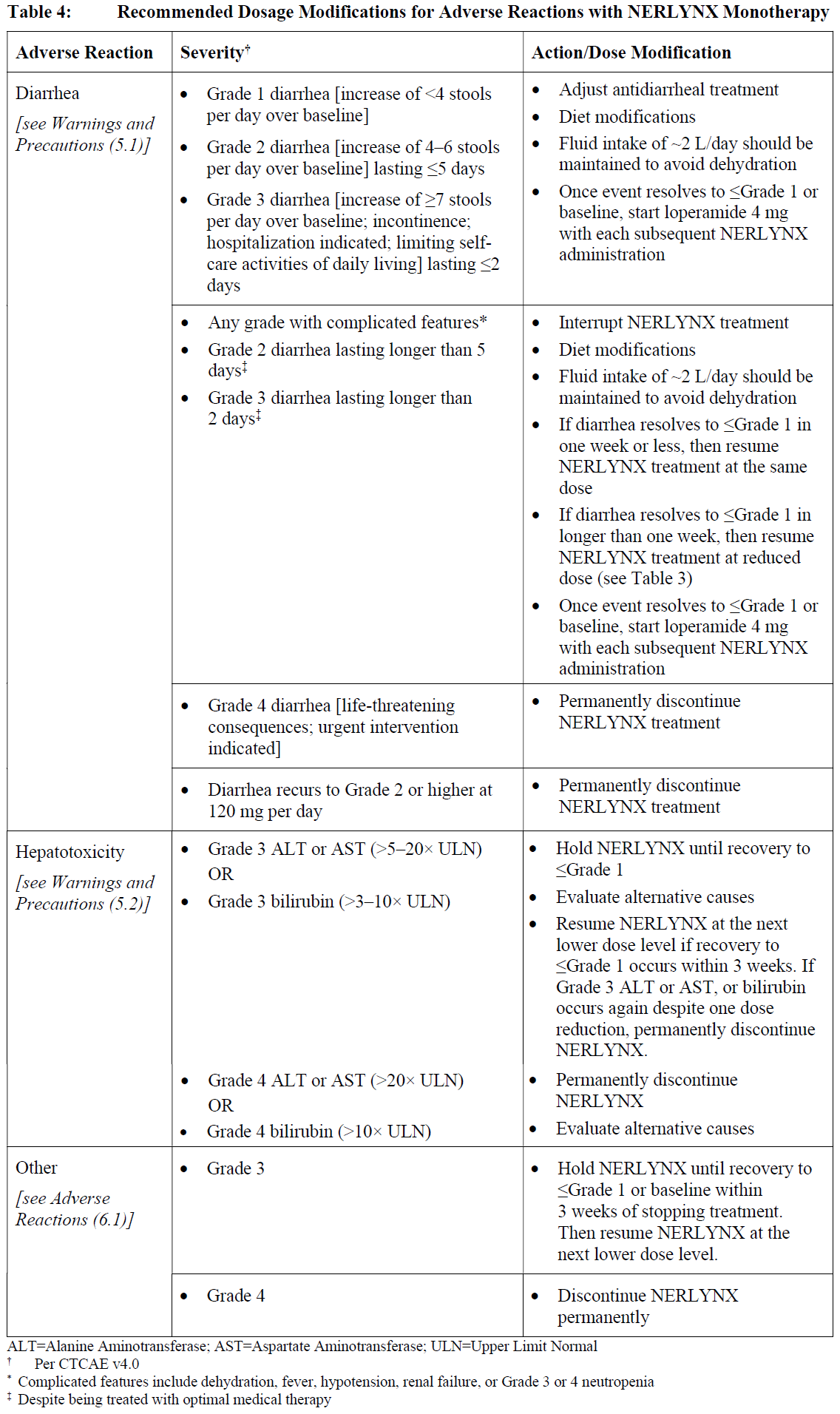 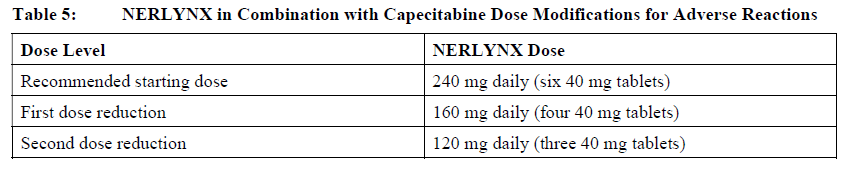 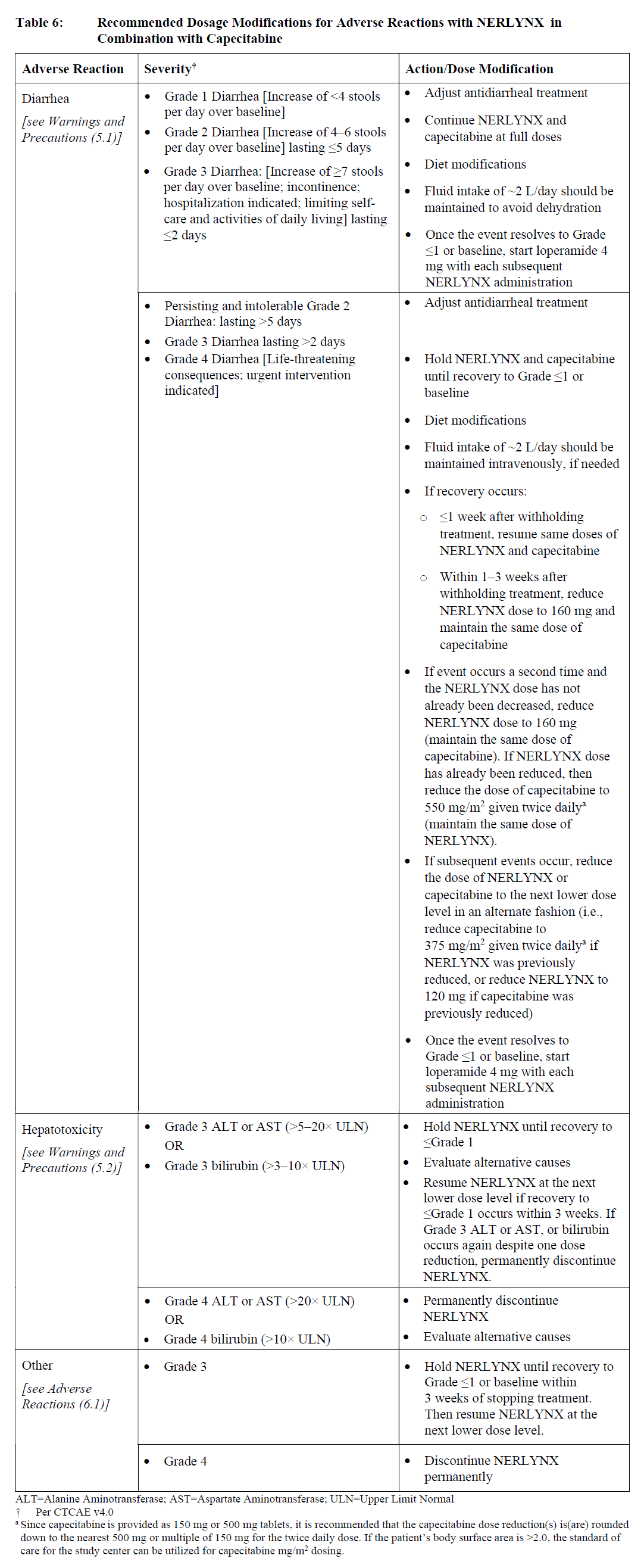 **2.4 Dosage Modifications for Hepatic Impairment** No dose modifications are recommended for patients with mild to moderate hepatic impairment (Child Pugh A or B). Treatment of patients with severe hepatic impairment (Child Pugh C) is not recommended \[ _see Use in Specific Populations (8.6) and Clinical Pharmacology (11.3)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. **2.5 Concomitant Use with Gastric Acid Reducing Agents** _Proton pump inhibitors (PPI):_ Avoid concomitant use with NERLYNX \[ _see Drug Interactions (7.1)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. _H2-receptor antagonists:_ Take NERLYNX at least 2 hours before the next dose of the H2-receptor antagonist or 10 hours after the H2-receptor antagonist \[ _see Drug Interactions (7.1)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. _Antacids:_ Separate dosing of NERLYNX by 3 hours after antacids \[ _see Drug Interactions (7.1)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_.
ORAL
Medical Information
**1 INDICATIONS AND USAGE** **1.1 Extended Adjuvant Treatment of Early-Stage Breast Cancer** NERLYNX as a single agent is indicated for the extended adjuvant treatment of adult patients with early stage HER2-overexpressed/amplified breast cancer, to follow adjuvant trastuzumab-based therapy \[ _see Clinical Studies (13.1)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_\]. **1.2 Advanced or Metastatic Breast Cancer** NERLYNX in combination with capecitabine is indicated for the treatment of adult patients with advanced or metastatic HER2-positive breast cancer who have received two or more prior anti-HER2 based regimens in the metastatic setting \[ _see Clinical Studies (13.2)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_\].
**4 CONTRAINDICATIONS** Hypersensitivity to the active substance or to any of the excipients contained in NERLYNX.
L01EH02
neratinib
Manufacturer Information
SPECIALISED THERAPEUTICS ASIA PTE. LTD.
Excella GmbH & Co. KG
Active Ingredients
Documents
Package Inserts
Nerlynx Film-Coated Tablets 40mg PI.pdf
Approved: December 7, 2022
