Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
SPRAY
**Dosage and Administration** A treatment session consists of nasal administration of SPRAVATO® and post-administration observation under the supervision of a healthcare professional. SPRAVATO® is for nasal use only. The nasal spray device is a single-use device that delivers a total of 28mg of esketamine in two sprays (one spray per nostril). To prevent loss of medication, the device should not be primed before use. It is intended for administration by the patient under the supervision of a healthcare professional, using 1 device (for a 28mg dose), 2 devices (for a 56mg dose) or 3 devices (for an 84mg dose), with a 5-minute rest between use of each device. **Blood pressure assessment before and after treatment** Assess blood pressure prior to dosing with SPRAVATO® (see _Warnings and Precautions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). If baseline blood pressure is elevated (e.g., >140 mmHg systolic, >90 mmHg diastolic), consider the risks of short term increases in blood pressure and benefit of SPRAVATO® treatment (see _Warnings and Precautions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Do not administer SPRAVATO® if an increase in blood pressure or intracranial pressure poses a serious risk (see _Contraindications_). After dosing with SPRAVATO®, reassess blood pressure at approximately 40 minutes and subsequently as clinically warranted. If blood pressure is decreasing and the patient appears clinically stable, the patient may leave at the end of the post-dose monitoring period; if not, continue to monitor (see _Warnings and Precautions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Since some patients may experience nausea and vomiting after administration of SPRAVATO®, patients should be advised not to eat for at least 2 hours before administration and not to drink liquids at least 30 minutes prior to administration (see _Adverse Reactions – Nausea and vomiting_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Patients who require a nasal corticosteroid or nasal decongestant on a dosing day should be advised not to administer these medications within 1 hour before administration of SPRAVATO®. For instructions to prepare the patient and for use of the nasal spray device, see _Instructions for Use and Handling_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. **Dosage – Adults** **Treatment-resistant Depression (TRD)** The dosage recommendations for SPRAVATO® for TRD are shown in Table 1. Dose adjustments should be made based on efficacy and tolerability to the previous dose. 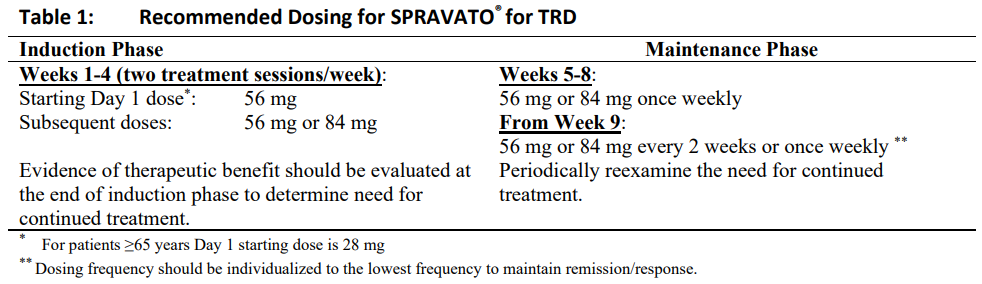 After depressive symptoms improve, treatment is recommended for at least 6 months. **Acute short-term treatment of psychiatric emergency due to Major Depressive Disorder** The recommended dosage for SPRAVATO® is 84 mg twice per week for 4 weeks. Dosage reduction to 56 mg should be made based on tolerability. After 4 weeks of treatment with SPRAVATO®, the oral antidepressant (AD) therapy should be continued, per clinical judgement. Patients who also have TRD should be evaluated to determine need for continued treatment with SPRAVATO® beyond 4 weeks. **Post-administration observation** During and after SPRAVATO® administration at each treatment session, a healthcare professional should observe the patient until the patient is stable based on clinical judgment. Rare cases of deep, delayed or prolonged sedation have been reported. Sedation typically showed an onset at around 15 minutes after dosing, with symptoms peaking at 30 to 45 minutes post-dose and resolving by 1.5 hours post-dose. Before SPRAVATO® administration, instruct patients not to engage in potentially hazardous activities, such as driving a motor vehicle or operating machinery until the next day after a restful sleep. (See _Warnings and Precautions – Effect on blood pressure, Potential for cognitive and motor impairment and effect on driving_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Missed treatment session(s)** If a patient misses treatment session(s) during the first 4 weeks of treatment, patients should continue their current dosing schedule. For patients with TRD who miss treatment session(s) during maintenance phase and have worsening of depression symptoms, per clinical judgement, consider returning to the previous dosing schedule (see Table 1). **Special populations** _**Pediatrics (17 years of age and younger)**_ The safety and efficacy of SPRAVATO® have not been established in patients aged 17 years and younger. _**Elderly (65 years of age and older)**_ In elderly patients the initial SPRAVATO® dose is 28 mg (Day 1, Starting Dose, see Table 1). Subsequent doses should be increased in increments of 28 mg up to 56 mg or 84 mg, based on efficacy and tolerability. SPRAVATO® has not been studied in elderly patients as acute short-term treatment of psychiatric emergency due to Major Depressive Disorder. _**Hepatic impairment**_ No dosage adjustment is necessary in patients with mild (Child-Pugh class A) or moderate (Child-Pugh class B) hepatic impairment. Use with caution in SPRAVATO®-treated patients with moderate hepatic impairment who may need to be monitored for adverse reactions for a longer period of time. SPRAVATO® has not been studied in patients with severe hepatic impairment (Child-Pugh class C). Use in this population is not recommended. (See _Pharmacokinetic Properties – Special populations, Hepatic impairment_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _**Japanese and Chinese patients with treatment-resistant depression**_ Efficacy of SPRAVATO® in Japanese and Chinese patients has not been established (See _Clinical Studies_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
NASAL
Medical Information
**Indications** SPRAVATO®, in combination with an oral antidepressant (SSRI or SNRI), is indicated for treatment-resistant depression (Major Depressive Disorder in adults who have not responded adequately to at least two different antidepressants of adequate dose and duration to treat the current depressive episode). SPRAVATO®, co-administered with oral antidepressant therapy, is indicated in adults with a moderate to severe episode of Major Depressive Disorder, as acute short-term treatment, for the rapid reduction of depressive symptoms, which according to clinical judgement constitute a psychiatric emergency. See _Clinical Studies_ for a description of the populations studied – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
**Contraindications** SPRAVATO® is contraindicated in patients for whom an increase in blood pressure or intracranial pressure poses a serious risk (see _Warnings and Precautions – Effect on blood pressure_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_): - Patients with known aneurysmal vascular disease (including intracranial, thoracic, or abdominal aorta, or peripheral arterial vessels) - Patients with known history of intracerebral hemorrhage SPRAVATO® is contraindicated in patients with a known hypersensitivity to esketamine, ketamine, or to any of the excipients.
N06AX27
esketamine
Manufacturer Information
JOHNSON & JOHNSON INTERNATIONAL (SINGAPORE) PTE. LTD.
Renaissance Lakewood LLC
Active Ingredients
Documents
Package Inserts
Spravato Nasal Spray PI.pdf
Approved: June 21, 2023
