Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**4 Dosage regimen and administration** Treatment with Scemblix should be initiated by a physician experienced in the use of anticancer therapies. **Dosage regimen** **General target population** **Ph+ CML-CP** The recommended total daily dose of Scemblix is 80 mg. Scemblix can be taken orally either as 80 mg once daily at approximately the same time each day, or as 40 mg twice daily at approximately 12-hour intervals. Patients changing from 40 mg twice daily to 80 mg once daily should start taking Scemblix once daily approximately 12 hours after the last twice-daily dose, and then continue at 80 mg once daily. Patients changing from 80 mg once daily to 40 mg twice daily should start taking Scemblix twice daily approximately 24 hours after the last once-daily dose and then continue at 40 mg twice daily at approximately 12-hour intervals. Any change in the dosage regimen is at the prescriber’s discretion, as necessary for the management of the patient. **Ph+ CML-CP harboring the T315I mutation** The recommended dose of Scemblix is 200 mg taken orally twice daily at approximately 12 hour intervals. Treatment with Scemblix should be continued as long as clinical benefit is observed or until unacceptable toxicity occurs. **Missed dose** **Once-daily dosage regimen:** If a Scemblix dose is missed by more than approximately 12 hours, it should be skipped, and the next dose should be taken as scheduled. **Twice-daily dosage regimens:** If a Scemblix dose is missed by more than approximately 6 hours, it should be skipped, and the next dose should be taken as scheduled. **Dose modifications** **Ph+ CML-CP** For the management of adverse drug reactions, Scemblix dose can be reduced based on individual safety and tolerability, as described in Table 4-1. If adverse drug reactions are effectively managed, Scemblix may be resumed as described in Table 4-1. Scemblix should be permanently discontinued in patients unable to tolerate a total daily dose of 40 mg. **PH+ CML-CP harboring the T315I mutation** For the management of adverse drug reactions, Scemblix dose can be reduced based on individual safety and tolerability, as described in Table 4-1. If adverse drug reactions are effectively managed, Scemblix may be resumed as described in Table 4-1. Scemblix should be permanently discontinued in patients unable to tolerate a dose of 160 mg twice daily. 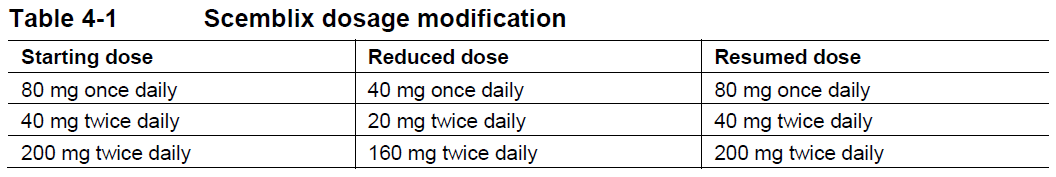 The recommended dosage modification for the management of selected adverse drug reactions is shown in Table 4-2. 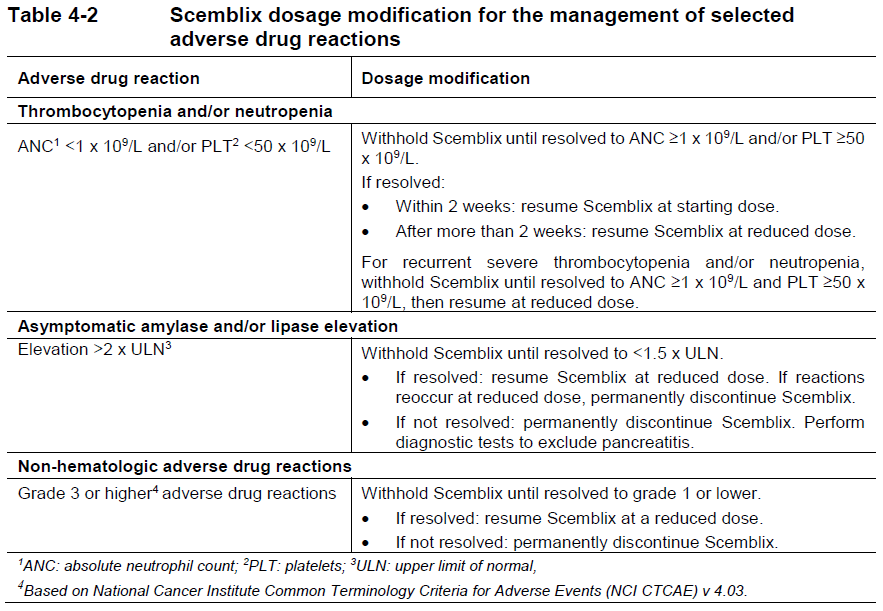 **Special populations** **Renal impairment** No dose adjustment is required in patients with mild, moderate, or severe renal impairment not requiring dialysis (absolute Glomerular Filtration Rate (aGFR) ≥15 mL/min) receiving Scemblix. Caution should be exercised in patients with severe renal impairment receiving Scemblix 200 mg twice daily dose. (see section 11 Clinical pharmacology – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Hepatic impairment** No dose adjustment is required in patients with mild, moderate, or severe hepatic impairment receiving Scemblix. Caution should be exercised in patients with severe hepatic impairment receiving Scemblix 200 mg twice daily dose. (see section 11 Clinical pharmacology – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Pediatric patients (below 18 years)** The safety and efficacy of Scemblix in pediatric patients (below 18 years) has not been established. **Geriatric patients (65 years of age or above)** No dose adjustment is required in patients 65 years of age or above. **Method of administration** Scemblix should be taken orally without food. Food consumption should be avoided for at least 2 hours before and 1 hour after taking Scemblix (see section 8 Interactions and 11 Clinical pharmacology – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Scemblix film-coated tablets should be swallowed whole and should not be broken, crushed, or chewed.
ORAL
Medical Information
**3 Indications** Scemblix® is indicated for the treatment of adult patients with: - Philadelphia chromosome-positive chronic myeloid leukemia (Ph+ CML) in chronic phase (CP) previously treated with two or more tyrosine kinase inhibitors (see section 12 Clinical studies – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). - Ph+ CML in CP harboring the T315I mutation.
**5 Contraindications** Hypersensitivity to the active substance or to any of the excipients listed in section 2 Description and composition – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
L01EA06
asciminib
Manufacturer Information
NOVARTIS (SINGAPORE) PTE LTD
Novartis Pharma Stein AG
Active Ingredients
Documents
Package Inserts
Scemblix Film-Coated Tablet PI.pdf
Approved: June 22, 2023
