Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, SUSPENSION, EXTENDED RELEASE
**Dosage and Administration** INVEGA HAFYERA® is to be used only after adequate treatment has been established with either the 1-month paliperidone palmitate injectable product for at least four months at dosages of 100 mg or 150 mg (see Table 1) or the 3-month paliperidone palmitate injectable product at dosages of 350 mg or 525 mg (see Table 2) for at least one injection cycle. In order to establish a consistent maintenance dose, it is recommended that the last two doses of the 1-month injection be the same dosage strength before starting INVEGA HAFYERA®. **Dosage** _**INVEGA HAFYERA® for patients adequately treated with 1-month paliperidone palmitate**_ Initiate INVEGA HAFYERA® at the time when the next 1-month paliperidone palmitate dose was to be scheduled with a INVEGA HAFYERA® dose based on the previous injection dose as shown in Table 1. INVEGA HAFYERA® may be administered up to 7 days before or after the monthly time point of the next scheduled paliperidone palmitate dose. 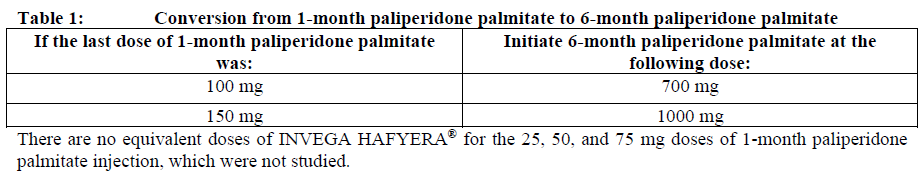 _**INVEGA HAFYERA® for patients adequately treated with 3-month paliperidone palmitate**_ Initiate INVEGA HAFYERA® at the time when the next 3-month paliperidone palmitate dose was to be scheduled with a INVEGA HAFYERA® dose based on the previous injection dose as shown in Table 2. INVEGA HAFYERA® may be administered up to 14 days before or after the 3-monthly time point of the next scheduled paliperidone palmitate dose. 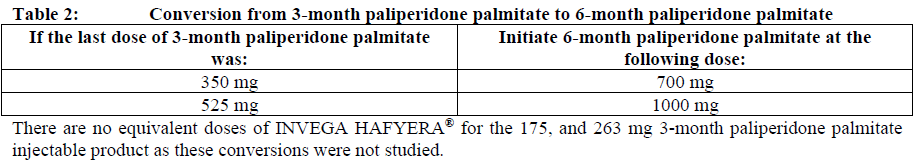 Following the initial dose, INVEGA HAFYERA® should be administered every 6 months. Missed doses of INVEGA HAFYERA® should be avoided, although injections given up to 2 weeks before or 3 weeks after the scheduled 6-month time point are not considered a missed dose. If needed, dose adjustment of INVEGA HAFYERA® can be made every 6 months between the dose levels of 700 mg and 1000 mg based on individual patient tolerability and/or efficacy. Due to the long-acting nature of 6-month paliperidone palmitate, the patient’s response to an adjusted dose may not be apparent for several months (see _Pharmacokinetic Properties_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). If the patient remains symptomatic, they should be managed according to clinical practice. **Missed dose** Dosing window To avoid a missed dose, patients may be given the injection up to 2 weeks before or 3 weeks after the scheduled 6-month time point. Missed dose over 6 months and 3 weeks, up to but less than 8 months since last injection If more than 6 months and 3 weeks up to but less than 8 months have elapsed since the last injection of INVEGA HAFYERA® do NOT administer the next dose of INVEGA HAFYERA®. Instead, use the re-initiation regimen shown in Table 3. 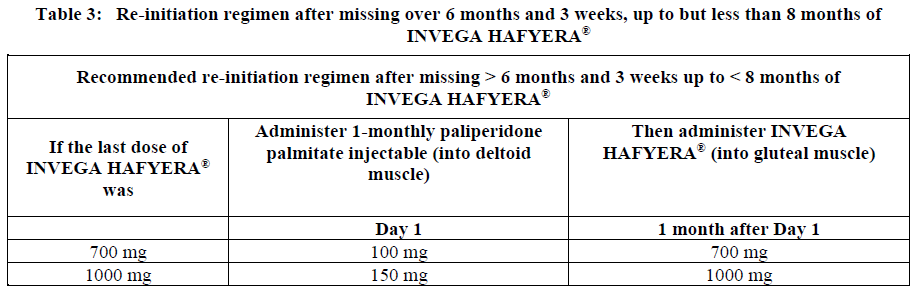 Missed dose 8 months up to and including 11 months since last injection If 8 months up to and including 11 months have elapsed since the last injection of INVEGA HAFYERA®, do NOT administer the next dose of INVEGA HAFYERA®. Instead, use the re-initiation regimen shown in Table 4. 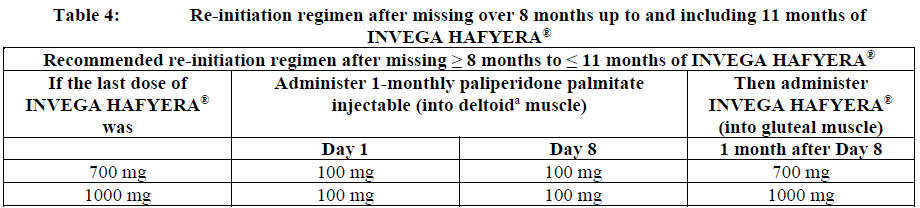 Missed doses over 11 months since last injection If more than 11 months have elapsed since the last injection of INVEGA HAFYERA®, re-initiate treatment with 1-month paliperidone palmitate injection as described in the prescribing information for that product. INVEGA HAFYERA® can then be resumed after the patient has been adequately treated with 1-month paliperidone palmitate injection for at least 4 months. To establish a consistent maintenance dose, it is recommended that the last two doses of 1-month paliperidone palmitate injection be the same dosage strength before re-starting INVEGA HAFYERA®. **Switching from other antipsychotic agents** INVEGA HAFYERA® is to be used only after the patient has been adequately treated with the 1-month paliperidone palmitate injectable product for at least 4 months or the 3-month paliperidone injectable product for one 3-month injection cycle (see _Indications_ and _Dosage and Administration_). If INVEGA HAFYERA® is discontinued, its prolonged-release characteristics must be considered. As recommended with other antipsychotic medications, the need for continuing existing extrapyramidal symptoms (EPS) medication should be re-evaluated periodically. **Transitioning from INVEGA HAFYERA® to the 3-Month Paliperidone Palmitate Injectable Product** Transitioning from INVEGA HAFYERA® to the 3-month paliperidone palmitate injectable product should be started 6 months after the last INVEGA HAFYERA® dose using the corresponding dose as shown in Table 5. The 3-month paliperidone palmitate injectable product should then continue, dosed at 3-monthly intervals. 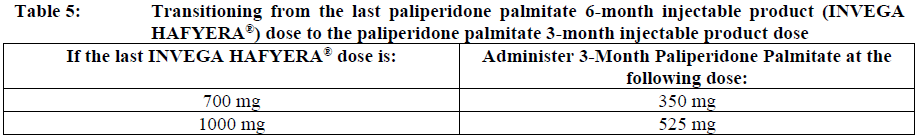 **Transitioning from INVEGA HAFYERA® to the 1-Month Paliperidone Palmitate Injectable Product** Transitioning from INVEGA HAFYERA® to the 1-month paliperidone palmitate injectable product should be started 6 months after the last INVEGA HAFYERA® dose, using the corresponding dose of 1-month paliperidone palmitate as shown in Table 6. The 1-month paliperidone palmitate injectable product should then continue dosed at monthly intervals. 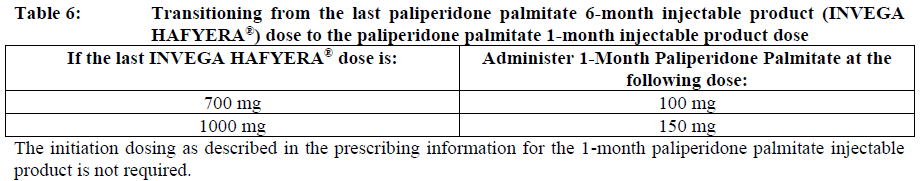 **Transitioning from INVEGA HAFYERA® to Oral Paliperidone Extended-Release Tablets** Transitioning from INVEGA HAFYERA® to oral paliperidone extended-release tablets should be started 6 months after the last INVEGA HAFYERA® dose and the daily dosing of the paliperidone extended-release tablets should be transitioned over the next several months as described in Table 7. Table 7 provides dose conversion regimens to allow patients previously stabilized on the dose levels of INVEGA HAFYERA® to attain similar paliperidone exposure with once daily paliperidone extended-release tablets. 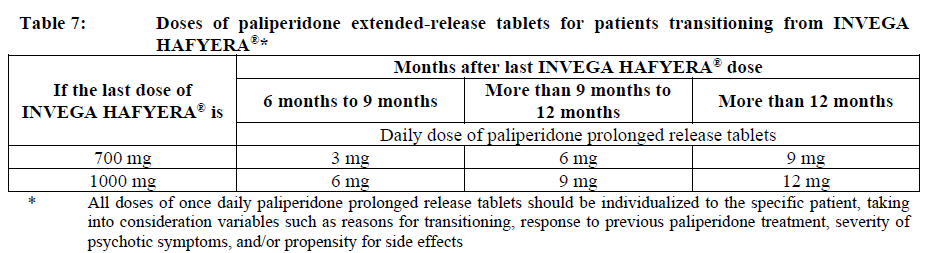 **Special populations** **_Pediatrics_** Safety and effectiveness of INVEGA HAFYERA® in patients < 18 years of age have not been studied. _**Elderly (65 years of age and older)**_ In general, recommended dosing of INVEGA HAFYERA® for elderly patients with normal renal function is the same as for younger adult patients with normal renal function. As elderly patients may have reduced renal function, see Renal impairment below for dosing recommendations in patients with renal impairment. _**Renal impairment**_ INVEGA HAFYERA® has not been systematically studied in patients with renal impairment (see _Pharmacokinetic Properties_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). For patients with mild renal impairment (creatinine clearance ≥ 50 to ≤ 80 mL/min), the dose should be adjusted and the patient stabilized using 1-month paliperidone palmitate injectable product. Transition to INVEGA HAFYERA® at the time when the next 1-month or 3-month paliperidone palmitate dose was to be scheduled with a INVEGA HAFYERA® dose based on the previous injection dose as shown in Table 1 and Table 2 respectively. The maximum recommended dose of INVEGA HAFYERA® in patients with mild renal impairment is 700 mg. INVEGA HAFYERA® is not recommended in patients with moderate or severe renal impairment (creatinine clearance < 50 mL/min). _**Hepatic impairment**_ INVEGA HAFYERA® has not been studied in patients with hepatic impairment. Based on a study with oral paliperidone, no dose adjustment is required in patients with mild or moderate hepatic impairment. Paliperidone has not been studied in patients with severe hepatic impairment. (See _Pharmacokinetic Properties_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) _**Other populations**_ No dose adjustment for INVEGA HAFYERA® is recommended based on gender, race, or smoking status. (For pregnant women and nursing mothers, see _Pregnancy, Breast-feeding and Fertility_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.) **Administration** INVEGA HAFYERA® should be administered once every 6 months. Each injection must be administered only by a healthcare professional. Parenteral drug products should be inspected visually for foreign matter and discoloration prior to administration. **This highly concentrated product requires specific steps to ensure complete resuspension:** - Holding the **syringe tip cap pointing up, shake** the syringe using a **very fast** up and down motion with a loose wrist for **at least 15 seconds** - **Rest briefly,** then **shake** again in the same way, **very fast** up and down motion with a loose wrist **for a further 15 seconds** **Proceed immediately to inject INVEGA HAFYERA®.** If more than **five minutes** passes before the injection is administered, shake the syringe again, as above to resuspend the medication. (See _Instructions for Use and Handling and Disposal_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). INVEGA HAFYERA® is for gluteal intramuscular use only. Do not administer by any other route. Avoid inadvertent injection into a blood vessel. Each injection must be administered only by a healthcare professional. Administer the dose in a single injection; do not administer the dose in divided injections. Inject slowly, deep into the upper-outer quadrant of the gluteal muscle Future injections should be alternated between the two gluteal muscles. Regardless of the patient’s weight, INVEGA HAFYERA® must be administered using only the thin wall 20 G, 1½-inch needles that is provided in the INVEGA HAFYERA® pack. Do not use needles from the 1-month or 3-month paliperidone palmitate injectable product packs or other commercially-available needles to reduce the risk of blockage. Since paliperidone is the active metabolite of risperidone, caution should be exercised when INVEGA HAFYERA® is coadministered with risperidone or with oral paliperidone for extended periods of time. Safety data involving concomitant use of INVEGA HAFYERA® with other antipsychotics is limited. _Incomplete Administration_ INVEGA HAFYERA® is a highly concentrated product that requires specific steps to ensure complete resuspension and prevent clogging of the needle during injection. Proper shaking can reduce the likelihood for an incomplete injection. Shipping and storing the carton in a horizontal orientation improves the ability to resuspend this highly concentrated product. Follow the details in the _Instructions for Use and Handling and Disposal_ to avoid an incomplete injection – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. However, in the event of an incompletely administered dose, do not re-inject the dose remaining in the syringe and do not administer another dose of INVEGA HAFYERA®. Closely monitor and treat the patient with oral supplementation as clinically appropriate until the next scheduled 6-month injection of INVEGA HAFYERA®.
INTRAMUSCULAR
Medical Information
**Indications** INVEGA HAFYERA®, a 6-month injection, is indicated for the treatment of schizophrenia in adult patients who have been adequately treated with the 1-month paliperidone palmitate injectable product for at least four months or the 3-month paliperidone palmitate injectable product following at least one 3-month injection cycle.
**Contraindications** INVEGA HAFYERA® is contraindicated in patients with a known hypersensitivity to paliperidone or to any of the components in the formulation. Since paliperidone is an active metabolite of risperidone, INVEGA HAFYERA® is contraindicated in patients with a known hypersensitivity to risperidone.
N05AX13
paliperidone
Manufacturer Information
JOHNSON & JOHNSON INTERNATIONAL (SINGAPORE) PTE. LTD.
Janssen Pharmaceutica NV
Active Ingredients
Documents
Package Inserts
Invega Hafyera_PI.pdf
Approved: March 8, 2023
