Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION
**Dosage and Administration** Particular attention should be paid to the specific dosing instructions for each proprietary Low Molecular Weight Heparin, as different units of measurement (units or mg) are used to express doses. Nadroparin should therefore not be used interchangeably with other low molecular weight heparins during ongoing treatment. In addition, care should be taken to use the correct formulation of nadroparin, either single or double strength, as this will affect the dosing regimen. Graduated syringes are intended for use when dose adjustment for body weight is necessary. Nadroparin is not intended for intramuscular injection. Platelet count must be monitored throughout nadroparin treatment ( _see Warnings and Precautions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Specific recommendations regarding the timing of nadroparin dosing surrounding spinal/epidural anaesthesia or spinal lumbar puncture should be followed ( _see Warnings and Precautions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _**Subcutaneous injection technique:**_ The usual site for subcutaneous injection is on the right or left side of the abdominal wall, but the thigh may be used as an alternative. To avoid loss of the solution when using pre-filled syringes, the air bubble should not be expelled from the syringe before the injection. The needle should be inserted perpendicularly into a pinched-up fold of skin which should be held gently but firmly until injection has been completed. The injection site should not be rubbed. **Populations** - **Adults** **PROPHYLAXIS OF THROMBOEMBOLIC DISORDERS** **_FRAXIPARINE_ ™ _INJECTION 950 INTERNATIONAL UNITS AXA/0.1ML_ \[Nadroparin calcium solution for injection 950 international units AXA/0.1ml\]** **_FRAXIPARINE_ ™ _MULTIDOSE INJECTION 9500 INTERNATIONAL UNITS AXA/ML_ \[Nadroparin calcium solution for injection 9500 international units AXA/ml\]** - _General Surgery_ The recommended dose of _FRAXIPARINE_ ™ is 0.3 ml (2,850 anti-Xa international units) administered subcutaneously 2 to 4 hours before surgery, and then once daily on subsequent days. Treatment should be continued for at least seven days, and throughout the risk period, until the patient is ambulant. - _Orthopaedic Surgery_ _FRAXIPARINE_ ™ is administered subcutaneously and the dose is adjusted for body weight according to the table below. This is based on a target dose of 38 anti-Xa international units per kg body weight, and is increased by 50% on the fourth post-operative day. The initial dose is administered 12 hours before surgery and a second dose 12 hours after the end of surgery. Treatment is then continued once daily throughout the risk period and until the patient is ambulant. The minimum treatment period is 10 days. 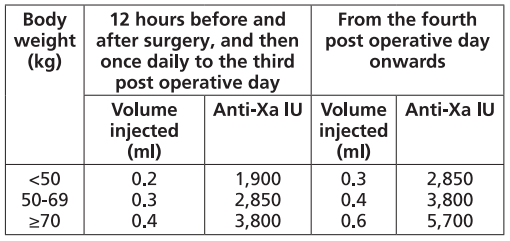 - _High-risk medical patients in intensive care (respiratory failure and/or respiratory infection and/or cardiac failure)_ _FRAXIPARINE_ ™ is administered subcutaneously once daily. The dose should be adjusted for body weight according to the table below. Treatment should be continued throughout the risk period of thromboembolism. 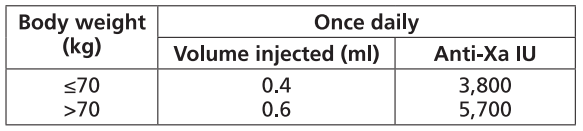 **TREATMENT OF THROMBOEMBOLIC DISORDERS** In the treatment of thromboembolic disorders, oral anti-coagulant therapy should be initiated as soon as possible unless contraindicated. Treatment with _FRAXIPARINE_ ™ should not be stopped before the International Normalised Ratio target is reached. **_FRAXIPARINE_ ™ _INJECTION 950 INTERNATIONAL UNITS AXA/0.1ML_ \[Nadroparin calcium solution for injection 950 international units AXA/0.1ml\]** **_FRAXIPARINE_ ™ _MULTIDOSE INJECTION 9500 INTERNATIONAL UNITS AXA/ML_ \[Nadroparin calcium solution for injection 9500 international units AXA/ml\]** It is recommended that _FRAXIPARINE_ ™ is administered subcutaneously twice daily (every 12 hours) for a usual duration of 10 days. The dose should be adjusted for body weight according to the table below, which is based on a target dose of 86 anti-Xa international units per kg body weight. 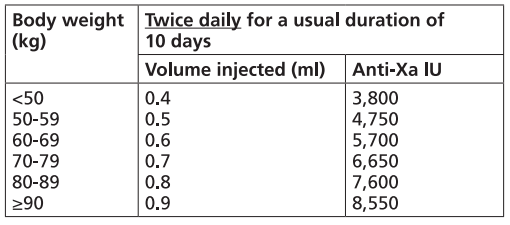 **_FRAXIPARINE FORTE_ ™ _INJECTION 19,000 INTERNATIONAL UNITS AXA/ML_ \[Nadroparin calcium Double Strength solution for injection 19,000 international units AXA/ml\]** It is recommended that _FRAXIPARINE FORTE_ ™ is administered subcutaneously once daily for a usual duration of 10 days. The dose is adjusted to the patient’s weight according to the table below, which is based on 171 anti-Xa international units per kg body weight. 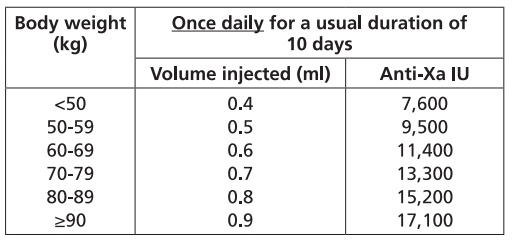 **PREVENTION OF CLOTTING DURING HAEMODIALYSIS** **_FRAXIPARINE_ ™ _INJECTION 950 INTERNATIONAL UNITS AXA/0.1ML_ \[Nadroparin calcium solution for injection 950 international units AXA/0.1ml\]** **_FRAXIPARINE_ ™ _MULTIDOSE INJECTION 9500 INTERNATIONAL UNITS AXA/ML_ \[Nadroparin calcium solution for injection 9500 international units AXA/ml\]** In the prevention of clotting during haemodialysis, the dose of _FRAXIPARINE_ ™ must be optimised for each individual patient, also taking into account the technical conditions of the dialysis. _FRAXIPARINE_ ™ is usually given as a single dose into the arterial line at the start of each session. For patients without increased risk of haemorrhage the following initial doses are suggested according to body weight and are usually sufficient for a four hour session: 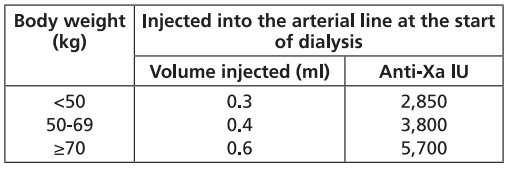 Doses should be halved in patients with an increased risk of haemorrhage. An additional smaller dose may be given during dialysis for sessions lasting longer than four hours. The dose in subsequent dialysis sessions should be adjusted as necessary according to the observed effect. Patients should be carefully monitored throughout each dialysis session for signs of bleeding or clotting in the dialysis circuit. **TREATMENT OF UNSTABLE ANGINA AND NON-Q WAVE MYOCARDIAL INFARCTION** **_FRAXIPARINE_ ™ _INJECTION 950 INTERNATIONAL UNITS AXA/0.1ML_ \[Nadroparin calcium solution for injection 950 international units AXA/0.1ml\]** **_FRAXIPARINE_ ™ _MULTIDOSE INJECTION 9500 INTERNATIONAL UNITS AXA/ML_ \[Nadroparin calcium solution for injection 9500 international units AXA/ml\]** It is recommended that _FRAXIPARINE_ ™ is administered subcutaneously twice daily (every 12 hours). The usual duration of treatment is six days. In clinical studies in patients with unstable angina and non-Q wave myocardial infarction, _FRAXIPARINE_ ™ was administered in combination with up to 325 mg aspirin per day. The initial dose is administered as a bolus injection intravenous (i.v.) and subsequent doses given by subcutaneous injection. The dose should be adjusted for body weight according to the table below, which is based on a target dose of 86 anti-Xa international units per kg body weight. 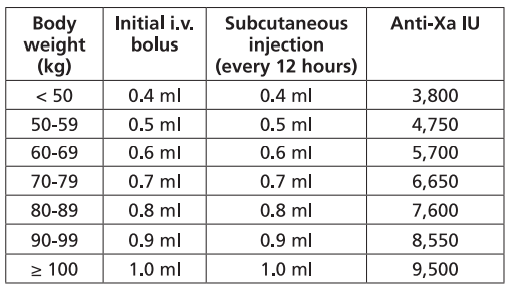 - **Children and Adolescents** Nadroparin is not recommended in children and adolescents as there are insufficient safety and efficacy data to establish dosage in patients aged less than 18 years. - **Elderly** No dosage adjustment is necessary in the elderly, unless renal function is impaired. It is recommended that renal function is assessed before initiating treatment ( _see Renal Impairment below, and Pharmacokinetics_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). - **Renal Impairment** _Prophylaxis of thromboembolic disorders_ Dose reduction is not required in patients with mild renal impairment (creatinine clearance greater than or equal to 50 ml/min). Moderate and severe renal impairment is associated with increased exposure to nadroparin. These patients are at increased risk of thromboembolism and haemorrhage. If a dose reduction is considered appropriate by the prescribing physician, taking into account the individual risk factors for haemorrhage and thromboembolism in patients with moderate renal impairment (creatinine clearance greater than or equal to 30 ml/min and less than 50 ml/min) the dose should be reduced by 25 to 33% (see Warnings and Precautions and Pharmacokinetics – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The dose should be reduced by 25 to 33% in patients with severe renal impairment (creatinine clearance less than 30 ml/min) (see Warnings and Precautions and Pharmacokinetics – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Treatment of thromboembolic disorders, unstable angina and non-Q wave myocardial infarction_ Dose reduction is not required in patients with mild renal impairment (creatinine clearance greater than or equal to 50 ml/min). Moderate and severe renal impairment is associated with increased exposure to nadroparin. These patients are at increased risk of thromboembolism and haemorrhage. If a dose reduction is considered appropriate by the prescribing physician, taking into account the individual risk factors for haemorrhage and thromboembolism in patients with moderate renal impairment (creatinine clearance greater than or equal to 30 ml/min and less than 50 ml/min) the dose should be reduced by 25 to 33% (see Warnings and Precautions and Pharmacokinetics – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Nadroparin is contraindicated in patients with severe renal impairment (see Warnings and Precautions and Pharmacokinetics – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). - **Hepatic impairment** There have been no studies conducted in patients with hepatic impairment.
SUBCUTANEOUS
Medical Information
**Indications** **_FRAXIPARINE_ ™ _INJECTION 950 INTERNATIONAL UNITS AXA/0.1ML_ \[Nadroparin calcium solution for injection 950 international units AXA/0.1ml\]** **_FRAXIPARINE_ ™ _MULTIDOSE INJECTION 9500 INTERNATIONAL UNITS AXA/ML_ \[Nadroparin calcium solution for injection 9500 international units AXA/ml\]** The prophylaxis of thromboembolic disorders, such as: - those associated with general or orthopaedic surgery - those in high risk medical patients (respiratory failure and/or respiratory infection and/or cardiac failure), hospitalised in intensive care unit The treatment of thromboembolic disorders. The prevention of clotting during haemodialysis. The treatment of unstable angina and non-Q wave myocardial infarction. **_FRAXIPARINE FORTE_ ™ _INJECTION 19,000 INTERNATIONAL UNITS AXA/ML_ \[Nadroparin calcium Double Strength solution for injection 19,000 international units AXA/ml\]** The treatment of thromboembolic disorders.
**Contraindications** Nadroparin is contraindicated in cases of: - hypersensitivity to nadroparin or any of the excipients of nadroparin injections - history of thrombocytopenia with nadroparin (see Warnings and Precautions – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) - active bleeding or increased risk of haemorrhage, in relation to haemostasis disorders, except for disseminated intravascular coagulation not induced by heparin - organic lesion likely to bleed (such as active peptic ulceration) - haemorrhagic cerebrovascular accident - acute infectious endocarditis - severe renal impairment (creatinine clearance less than 30 ml/min) in patients receiving treatment for thromboembolic disorders, unstable angina, and non-Q wave myocardial infarction - multi-dose vials contain benzyl alcohol and therefore should not be used in children under 3 years
B01AB06
nadroparin
Manufacturer Information
DCH AURIGA SINGAPORE
ASPEN NOTRE DAME DE BONDEVILLE
Active Ingredients
Documents
Package Inserts
Fraxiparine and Fraxiparine Forte injection PI.pdf
Approved: March 9, 2022
