Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
IMPLANT
**4.2 Posology and method of administration** Posology The indication for treatment should be established and the long-term therapy monitoring carried out by physicians experienced in tumour therapy. _**LEUPRORELIN SANDOZ IMPLANT IN SYRINGE 3.6 MG**_ The recommended dose is a single dose of 3.6 mg leuprorelin once monthly. After the second administration, if, in exceptional cases, the date of administration is postponed by up to 2 weeks, the therapeutic effect is not expected to be impaired in the majority of patients (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _**LEUPRORELIN SANDOZ IMPLANT IN SYRINGE 5 MG**_ The recommended dose is a single-dose of 5 mg leuprorelin once every 3 months. If, in exceptional cases, the date of administration is postponed by up to 4 weeks, the therapeutic effect is not expected to be impaired in the majority of patients (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Special populations No dose adjustment is necessary for patients with renal or hepatic impairment, or in older people. LEUPRORELIN SANDOZ IMPLANT IN SYRINGE is contraindicated in children and adolescents (see section 4.3). Monitoring advice Response to treatment with LEUPRORELIN SANDOZ IMPLANT IN SYRINGE should be monitored by measuring serum concentrations of prostate-specific antigen and testosterone periodically. Therapy of advanced prostate carcinoma with LEUPRORELIN SANDOZ IMPLANT IN SYRINGE is generally a long-term therapy. Method of administration One implant is injected subcutaneously into the anterior abdominal wall, where it forms a drug delivery depot to hydrolytically degrade, releasing the active substance. It provides continuous release of leuprorelin for one month for LEUPRORELIN SANDOZ IMPLANT IN SYRINGE 3.6 MG and for three months for LEUPRORELIN SANDOZ IMPLANT IN SYRINGE 5 MG. LEUPRORELIN SANDOZ IMPLANT IN SYRINGE is for use in one patient on one occasion only. It contains no antimicrobial preservative. Before injection, a local anaesthetic may be given. It is recommended that administration of an anti-androgen is started as adjunctive therapy about 5 days before starting LEUPRORELIN SANDOZ IMPLANT IN SYRINGE (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Instructions for use 1. Disinfect the injection site on the anterior abdominal wall below the navel line. 2. Remove the applicator from the sterile bag and check that the implant is visible in the repository (see framed area). For verifying, view the applicator against a light or gently shake it. 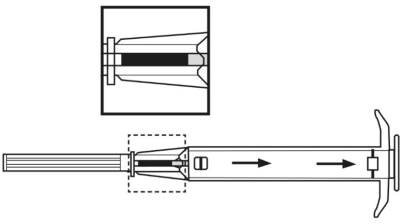 3. Pull the plunger of the applicator **completely backwards until you can see a complete line in the second window**. **Please note:** The plunger can only be pushed forward to inject the implant if it has been previously **pulled back completely!** 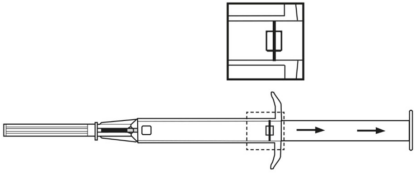 4. Remove the protective cap from the needle. 5. Hold the main body of the applicator with one hand. With the other hand pinch the patient’s skin of the anterior abdominal wall below the navel line. See illustration. With the needle **opening facing upwards, insert the whole needle**. Do this at a slight angle, almost parallel to the skin into the subcutaneous tissue. 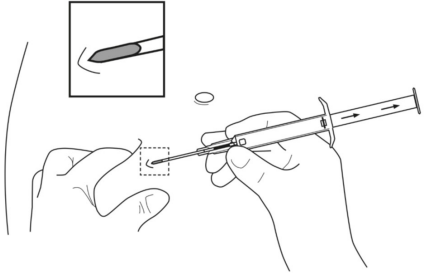 6. Carefully **pull** the applicator approximately 1 cm backwards. This creates the puncture canal for the implant. 7. Inject the implant into the puncture canal by pushing the plunger **completely** forwards until it snaps into place and you **hear a click**. 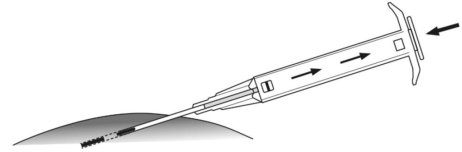 8. Withdraw the needle. To ensure that the implant has been injected correctly, check that the tip (light blue tip for 5mg implant/ white tip for 3.6mg implant) of the plunger is visible at the tip of the needle. 
SUBCUTANEOUS
Medical Information
**4.1 Therapeutic indications** Palliative treatment of patients with advanced hormone-dependent prostate carcinoma.
**4.3 Contraindications** Hypersensitivity to the active substance or to any of the excipients, or to other GnRH analogues. Confirmed hormone independence of the carcinoma. Leuprorelin is contraindicated in women and paediatric patients.
L02AE02
leuprorelin
Manufacturer Information
SANDOZ SINGAPORE PTE. LTD.
EVER Pharma Jena GmbH
Synergy Health Radeberg GmbH (Terminal sterilisation)
Active Ingredients
Documents
Package Inserts
Leuprorelin Sandoz PI.pdf
Approved: March 21, 2023
