Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION
**DOSAGE AND ADMINISTRATION** **For adult usage:** Factor IX complex, Proflnine® should be administered intravenously, promptly following reconstitution with the supplied diluent. Although Profilnine® is stable for at least three (3) hours at room temperature after reconstitution, prompt administration is recommended to avoid the ill effect of any inadvertent bacterial contamination occurring during reconstitution. Administer at room temperature, do not refrigerate after reconstitution and discard any unused contents. A 1.0% increase in factor IX (0.01 international units)/international units administered/kg can be expected.1, 5 The amount of Profilnine® required to establish hemostasis will vary with each patient and depends on the circumstances. The following formula may be used as a guide in determining the number of units to be administered: 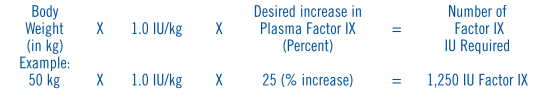 In normal clinical practice there is variability among patients and their clinical condition. Therefore, the factor IX level of each patient should be monitored frequently during replacement therapy. Mild to moderate hemorrhages may usually be treated with a single administration sufficient to raise the plasma factor IX level to 20 to 30 percent. In the event of more serious hemorrhage, the patient's plasma factor IX level should be raised to 30 to 50 percent, Infusions are generally required daily. Surgery in patients with factor IX deficiency requires that the factor IX level should be raised to 30 to 50 percent for at least one week following operation. For dental extractions, the factor IX level should be raised to 50 percent immediately prior to the procedure; additional factor IX complex may be given if bleeding recurs. **For pediatric usage:** See PRECAUTIONS – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. * * * 1\. Menache, D., Roberts, H.R. Summary report and recommendations of the task force members and consultants. _Thromb Diath Haemorrh_ 33:645–647, 1975. 5\. Data on file at Grifols Biologicals LLC
INTRAVENOUS
Medical Information
**INDICATIONS AND USAGE** Profilnine® is indicated for the prevention and control of bleeding in patients with factor IX deficiency due to hemophilia B. Proflnine® contains non-therapeutic levels of factor VII, and is not indicated for use in the treatment of factor VII deficiency.
**CONTRAINDICATIONS** None known.
B02BD04
coagulation factor IX
Manufacturer Information
GRIFOLS ASIA PACIFIC PTE. LTD.
GRIFOLS BIOLOGICALS LLC.
INSTITUTO GRIFOLS, S.A. (manufacturer for WFI)
ROVI PHARMA INDUSTRIAL SERVICES, S.A. (alternate manufacturer for WFI)
Active Ingredients
Documents
Package Inserts
1.4.3 Revised Artwork for Profilnine PI.pdf
Approved: March 14, 2019
