Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, EFFERVESCENT
**POSOLOGY AND METHOD OF ADMINISTRATION** Posology **Adults** The dose of ENDOMETRIN® is 100 mg administered vaginally two or three times daily starting at oocyte retrieval and continuing for up to 10 weeks total duration (or 12 weeks of gestation). Specific populations may derive greater benefits from BID or TID dosing regimen and the clinician can tailor treatment to the patient (see “Clinical efficacy and safety” section – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Serum progesterone levels may be measured 7 days post fertilization and used to guide therapy. **Paediatric population** There is no relevant use of ENDOMETRIN® in the paediatric population. **Elderly** No clinical data have been collected in patients over age 65. **Use in special populations** There is no experience with use of ENDOMETRIN® in patients with impaired liver or renal function. Method of Administration ENDOMETRIN® is to be placed directly into the vagina by the applicator provided. **Instructions for Use** 1. Unwrap the applicator. 2. Put one tablet in the space provided at the end of the applicator. The tablet should fit securely and not fall out. 3. The applicator with the tablet may be inserted into the vagina while you are standing, sitting, or when lying on your back with your knees bent. Gently insert the thin end of the applicator well into the vagina. 4. Push the plunger to release the tablet. Remove the applicator and rinse it thoroughly in warm running water, wipe dry with a soft tissue and keep the applicator for subsequent use. 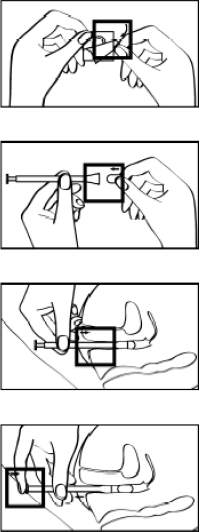
VAGINAL
Medical Information
**THERAPEUTIC INDICATIONS** ENDOMETRIN® is indicated for luteal support as part of an Assisted Reproductive Technology (ART) treatment program for infertile women.
**CONTRAINDICATIONS** ENDOMETRIN® should not be used in individuals with any of the following conditions: - Hypersensitivity to the active substance or to any of the excipients listed in section QUALITATIVE AND QUANTITATIVE COMPOSITION – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ - Undiagnosed vaginal bleeding - Known missed abortion or ectopic pregnancy - Severe hepatic dysfunction or disease - Known or suspected breast or genital tract cancer - Active arterial or venous thromboembolism or severe thrombophlebitis, or a history of these events - Porphyria
G03DA04
progesterone
Manufacturer Information
FERRING PHARMACEUTICALS PRIVATE LIMITED
QPharma AB
Active Ingredients
Documents
Package Inserts
Endometrin Vaginal Tablet PI.pdf
Approved: May 12, 2016
