Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INFUSION, SOLUTION CONCENTRATE
**2.2 DOSAGE AND ADMINISTRATION** **General** Tecentriq must be administered as an intravenous infusion under the supervision of a qualified healthcare professional. Do not administer as an IV push or bolus. Do not co-administer other medicinal products through the same infusion line. Substitution by any other biological medicinal product requires the consent of the prescribing physician. The safety and efficacy of alternating or switching between Tecentriq and products that are biosimilar but not deemed interchangeable to Tecentriq has not been established. Therefore, the benefit/risk of alternating or switching need to be carefully considered. The initial dose of Tecentriq must be administered over 60 minutes. If the first infusion is tolerated, all subsequent infusions may be administered over 30 minutes. The recommended dose of Tecentriq in monotherapy or combination therapy is: - 840 mg administered by IV infusion every 2 weeks, or - 1200 mg administered by IV infusion every 3 weeks, or - 1680 mg administered by IV infusion every 4 weeks. **Tecentriq monotherapy** _Early-stage NSCLC, 1L metastatic NSCLC_ Patients should be selected for treatment based on the tumor expression of PD-L1 confirmed by a validated test (see section 3.1.2 Clinical / Efficacy Studies – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Tecentriq combination therapy** For the use of Tecentriq in combination therapy, please also refer to the full prescribing information for the combination product. Tecentriq should be administered prior to the combination therapy if given on the same day. _**1L non-squamous metastatic NSCLC**_ _Tecentriq in combination with Avastin, paclitaxel, and carboplatin_ During the induction phase, Tecentriq is administered according to its dosing schedules by intravenous (IV) infusion, and Avastin, paclitaxel, and carboplatin are administered every 3 weeks for four or six cycles. The induction phase is followed by a maintenance phase without chemotherapy in which Tecentriq is administered according to its dosing schedules by IV infusion, and Avastin is administered every 3 weeks. _Tecentriq in combination with nab-paclitaxel and carboplatin_ During the induction phase, Tecentriq is administered according to its dosing schedules by IV infusion, and nab-paclitaxel and carboplatin are administered every 3 weeks for four or six cycles. For each 21-day cycle, nab-paclitaxel and carboplatin are administered on day 1. In addition, nab-paclitaxel is administered on days 8 and 15. The induction phase is followed by a maintenance phase without chemotherapy in which Tecentriq is administered according to its dosing schedule. _**1L ES-SCLC**_ _Tecentriq in combination with carboplatin and etoposide_ During the induction phase, Tecentriq is administered according to its dosing schedules by IV infusion, and carboplatin and etoposide are administered by IV infusion every three weeks for four cycles. Carboplatin and etoposide are administered on day 1 of each cycle, and etoposide is also administered on days 2 and 3. The induction phase is followed by a maintenance phase without chemotherapy in which Tecentriq is administered according to its dosing schedules by IV infusion. _**1L TNBC**_ _Tecentriq in combination with nab-paclitaxel_ Tecentriq is administered according to its dosing schedules by IV infusion and 100 mg/m2 nab-paclitaxel is administered on days 1, 8 and 15 during each 28-day cycle. Patients should be selected for treatment based on the tumor expression of PD-L1 confirmed by a validated test (see section 3.1.2 Clinical / Efficacy Studies – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **_HCC_** _Tecentriq in combination with Avastin_ Tecentriq is administered according to its dosing schedules by IV infusion, and Avastin 15 mg/kg is administered every 3 weeks. **Duration of Treatment** Patients are treated with Tecentriq until loss of clinical benefit (see section 3.1.2 Clinical / Efficacy Studies – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) or unacceptable toxicity. _1L TNBC_ Patients are treated with Tecentriq until disease progression or unacceptable toxicity. (see section 3.1.2 Clinical / Efficacy Studies – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) _Early-stage NSCLC_ Patients are treated with Tecentriq for 1 year unless there is disease recurrence or unacceptable toxicity (see section 3.1.2 Clinical / Efficacy Studies – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Delayed or Missed Doses** If a planned dose of Tecentriq is missed, it should be administered as soon as possible. The schedule of administration should be adjusted to maintain the appropriate interval between doses. **Dose Modifications** No dose reductions of Tecentriq are recommended. **Dose modifications for immune-mediated adverse reactions** Recommendations for specific adverse drug reactions (see sections 2.4.1 Warnings and Precautions, General and 2.6.1 Undesirable Effects, Clinical Trials – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) are presented in Table 1. 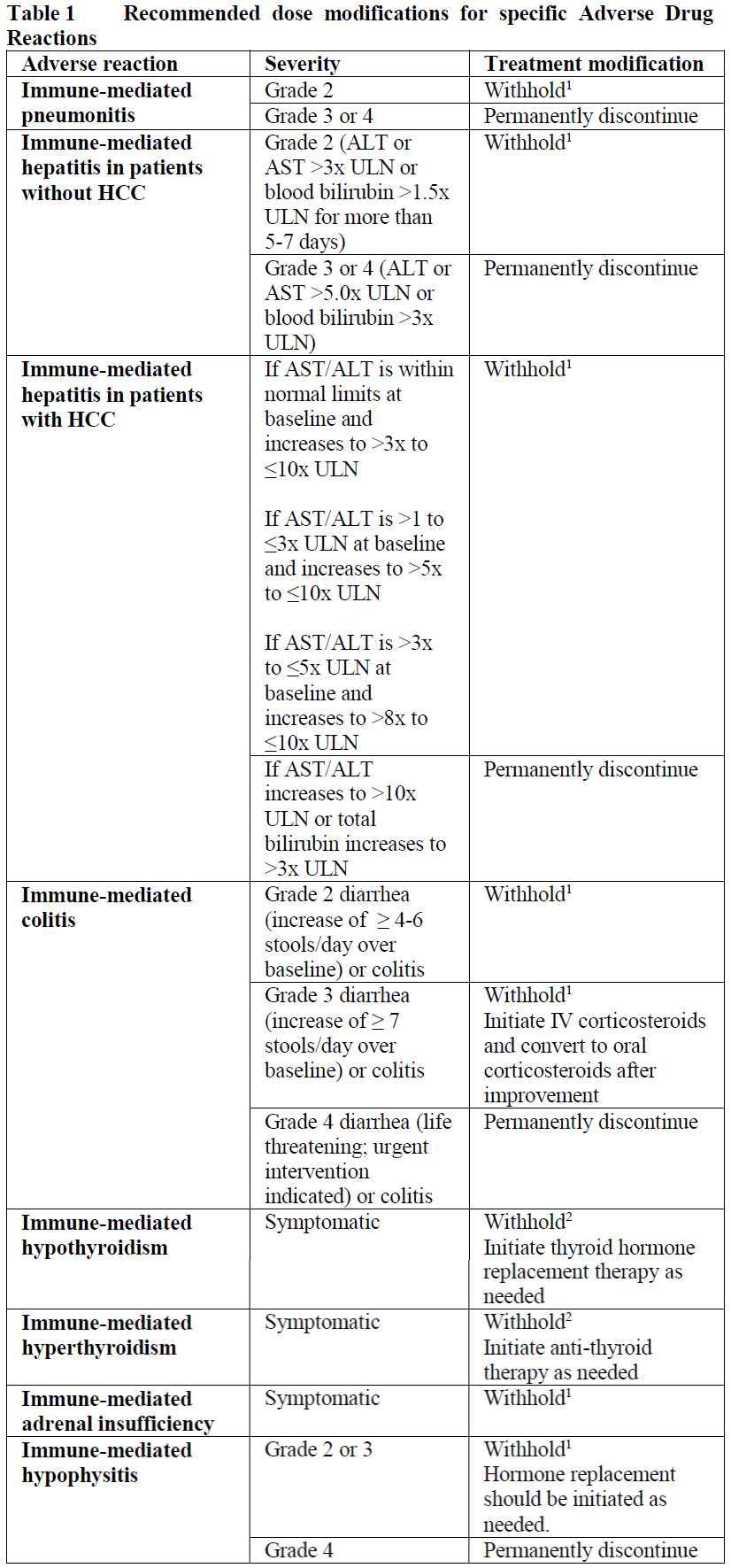 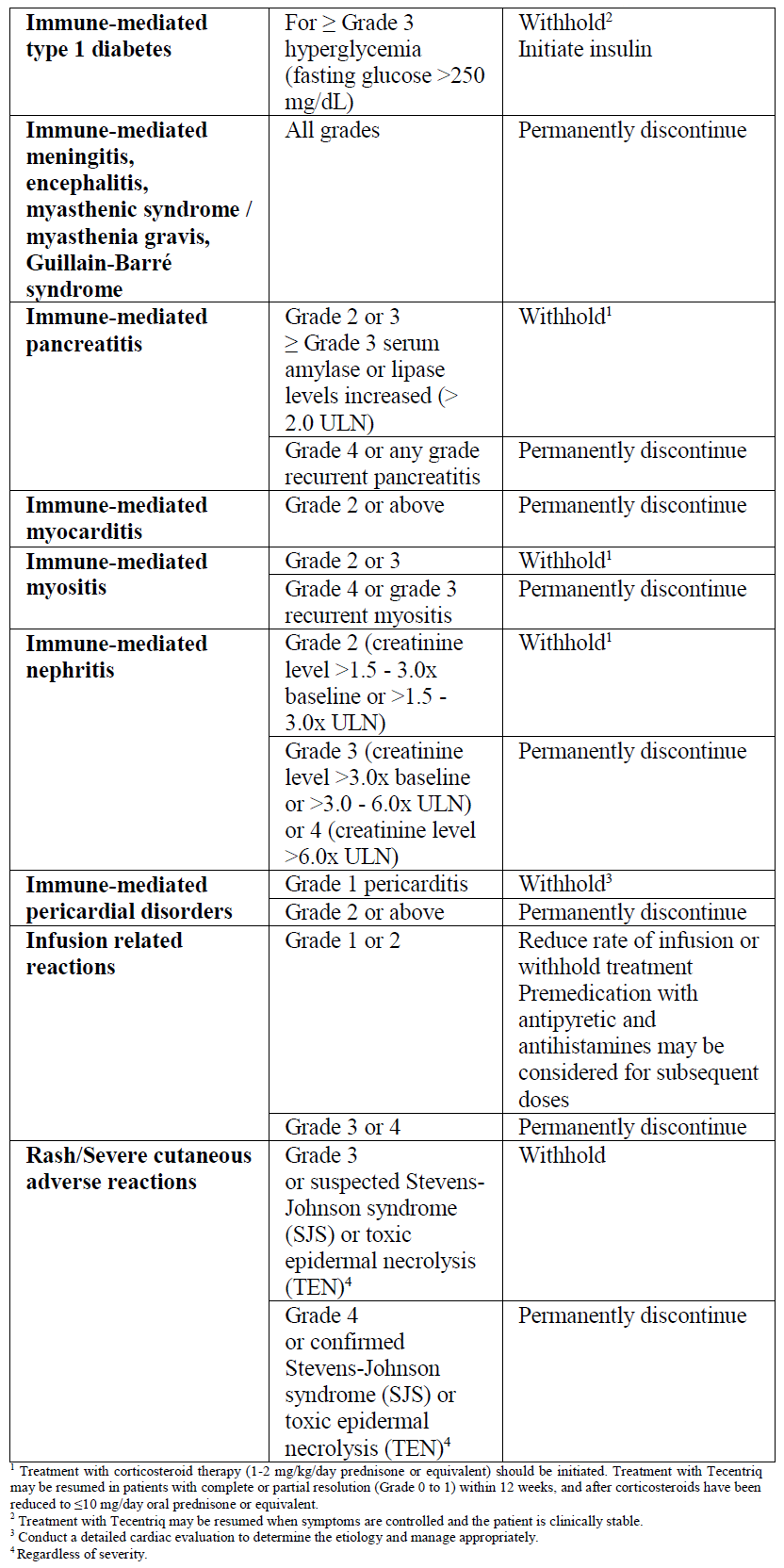 For other immune-mediated reactions, based on the type and severity of the reaction, treatment with Tecentriq should be withheld for Grades 2 or 3 immune-mediated adverse reactions and corticosteroid therapy (1–2 mg/kg/day prednisone or equivalent) should be initiated. If symptoms improve to ≤ Grade 1, taper corticosteroids as clinically indicated. Treatment with Tecentriq may be resumed if the event improves to ≤ Grade 1 within 12 weeks, and corticosteroids have been reduced to ≤ 10 mg oral prednisone or equivalent per day. Treatment with Tecentriq should be permanently discontinued for Grade 4 immune-mediated adverse reactions, or when unable to reduce corticosteroid dose to the equivalent of ≤ 10 mg prednisone per day within 12 weeks after onset. **2.2.1 Special Dosage Instructions** **Pediatric Use** The safety and efficacy of Tecentriq in children and adolescents below 18 years of age have not been established. (see section 2.5.4 Pediatric Use, and 3.2.5 Pharmacokinetics in Special Populations – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) **Geriatric Use** Based on a population pharmacokinetic analysis, no dose adjustment of Tecentriq is required in patients ≥ 65 years of age (see sections 2.5.5 Geriatric Use, and 3.2.5 Pharmacokinetics in Special Populations – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Renal Impairment** Based on a population pharmacokinetic analysis, no dose adjustment is required in patients with renal impairment (see section 3.2.5 Pharmacokinetics in Special Populations – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Hepatic impairment** Based on a population pharmacokinetic analysis, no dose adjustment is required for patients with mild or moderate hepatic impairment. There are no data in patients with severe hepatic impairment (see section 3.2.5 Pharmacokinetics in Special Populations – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
INTRAVENOUS
Medical Information
**2.1 THERAPEUTIC INDICATION(S)** **Early-stage non-small cell lung cancer** Tecentriq as monotherapy is indicated as adjuvant treatment following complete resection for adult patients with Stage II to IIIA (7th edition of the UICC/AJCC staging system) non-small cell lung cancer (NSCLC) whose tumours have PD-L1 expression on ≥ 50% of tumour cells (TC) and whose disease has not progressed following platinum-based adjuvant chemotherapy (see Section 3.1.2 Clinical / Efficacy Studies – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Metastatic non-small cell lung cancer** Tecentriq, in combination with Avastin, paclitaxel and carboplatin, is indicated for the treatment of patients with metastatic non-squamous non-small cell lung cancer (NSCLC) who had not received prior chemotherapy. Tecentriq as monotherapy is indicated for the treatment of patients with metastatic non-small cell lung cancer (NSCLC) who have disease progression during or following platinum-containing chemotherapy. Patients with EGFR or ALK genomic tumor aberrations should have disease progression on approved therapy for these aberrations prior to receiving Tecentriq. Tecentriq, in combination with nab-paclitaxel and carboplatin, is indicated for first-line treatment of patients with metastatic non-squamous NSCLC who do not have EGFR or ALK genomic tumor aberrations. Tecentriq as monotherapy is indicated for the first-line treatment of patients with metastatic NSCLC whose tumors have a PD-L1 expression ≥ 50% tumor cells (TC) or ≥ 10% tumor-infiltrating immune cells (IC) and who do not have EGFR or ALK genomic tumor aberrations. **Small cell lung cancer** Tecentriq, in combination with carboplatin and etoposide, is indicated for the first-line treatment of patients with extensive-stage small cell lung cancer (ES-SCLC). **Triple-negative breast cancer** Tecentriq, in combination with nab-paclitaxel, is indicated for the treatment of patients with unresectable locally advanced or metastatic triple-negative breast cancer (TNBC) whose tumors have PD-L1 expression of ≥1% on IC, and who have not received prior chemotherapy for metastatic disease. **Hepatocellular carcinoma** Tecentriq, in combination with Avastin, is indicated for the treatment of patients with unresectable hepatocellular carcinoma (HCC) who have not received prior systemic therapy.
**2.3 CONTRAINDICATIONS** Tecentriq is contraindicated in patients with a known hypersensitivity to atezolizumab or any of the excipients.
L01FF05
atezolizumab
Manufacturer Information
ROCHE SINGAPORE PTE. LTD.
Roche Diagnostics GmbH
F. Hoffmann-La Roche Ltd
Active Ingredients
Documents
Package Inserts
Tecentriq Infusion PI.pdf
Approved: March 10, 2023
