Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, SOLUTION
**4.2 Posology and method of administration** **Posology** Adults The starting dose is 0.6 mg once daily. The dose should be increased to 3.0 mg once daily in increments of 0.6 mg with at least one week intervals to improve gastro-intestinal tolerability (see table 2). If escalation to the next dose step is not tolerated for two consecutive weeks, consider discontinuing treatment. Daily doses higher than 3.0 mg are not recommended. 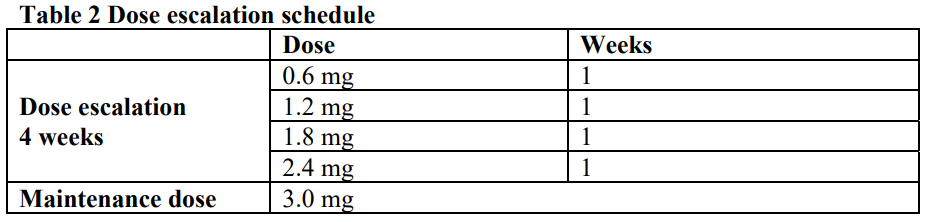 Adolescents (≥12 years) For adolescents from the age of 12 to below 18 years old a similar dose escalation schedule as for adults should be applied (see table 2). The dose should be increased until 3.0 mg (maintenance dose) or maximum tolerated dose has been reached. Daily doses higher than 3.0 mg are not recommended. Missed doses If a dose is missed within 12 hours from when it is usually taken, the patient should take the dose as soon as possible. If there is less than 12 hours to the next dose, the patient should not take the missed dose and resume the once-daily regimen with the next scheduled dose. An extra dose or increase in dose should not be taken to make up for the missed dose. Patients with type 2 diabetes mellitus Saxenda® should not be used in combination with another GLP-1 receptor agonist. When initiating Saxenda®, it should be considered to reduce the dose of concomitantly administered insulin or insulin secretagogues (such as sulfonylureas) to reduce the risk of hypoglycaemia. Blood glucose self-monitoring is necessary to adjust the dose of insulin or insulin-secretagogues (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Special populations _Elderly (≥65 years old)_ No dose adjustment is required based on age. Therapeutic experience in patients ≥75 years of age is limited and use in these patients is not recommended (see sections 4.4 and 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Renal impairment_ No dose adjustment is required for patients with mild or moderate renal impairment (creatinine clearance ≥30 mL/min). Saxenda® is not recommended for use in patients with severe renal impairment (creatinine clearance <30 mL/min) including patients with end-stage renal disease (see sections 4.4, 4.8 and 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Hepatic impairment_ No dose adjustment is recommended for patients with mild or moderate hepatic impairment. Saxenda® is not recommended for use in patients with severe hepatic impairment and should be used cautiously in patients with mild or moderate hepatic impairment (see sections 4.4 and 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Paediatric population_ No dose adjustment is required for adolescents from the age of 12 years and above. The safety and efficacy of Saxenda® in children below 12 years of age has not been established (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Method of administration** Saxenda® is for subcutaneous use only. It must not be administered intravenously or intramuscularly. Saxenda® is administered once daily at any time, independent of meals. It should be injected in the abdomen, thigh or upper arm. The injection site and timing can be changed without dose adjustment. However, it is preferable that Saxenda® is injected around the same time of the day, when the most convenient time of the day has been chosen. For further instructions on administration, see section 6.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
SUBCUTANEOUS
Medical Information
**4.1 Therapeutic indications** Adults Saxenda® is indicated as an adjunct to a reduced-calorie diet and increased physical activity for weight management in adult patients with an initial Body Mass Index (BMI) of - ≥30 kg/m2 (obesity), or - ≥27 kg/m2 to <30 kg/m2 (overweight) in the presence of at least one weight-related comorbidity such as dysglycaemia (prediabetes or type 2 diabetes mellitus), hypertension, dyslipidaemia or obstructive sleep apnoea. Treatment with Saxenda® should be discontinued after 12 weeks on the 3.0 mg/day dose if patients have not lost at least 5% of their initial body weight. Adolescents (≥12 years) Saxenda® can be used as an adjunct to a healthy nutrition and increased physical activity for weight management in adolescent patients from the age of 12 years and above with: - an inadequate response to reduced calorie diet and increased physical activity alone, and - obesity (BMI corresponding to ≥30 kg/m2 for adults by international cut-off points)\* and - body weight above 60 kg. Limitations of Use: The safety and effectiveness of Saxenda® in pediatric patients with type 2 diabetes have not been established. Treatment with Saxenda® should be discontinued and re-evaluated if patients have not lost at least 4% of their BMI or BMI z score after 12 weeks on the 3.0 mg/day or maximum tolerated dose. \*IOTF BMI cut-off points for obesity by sex between 12–18 years (see table 1): 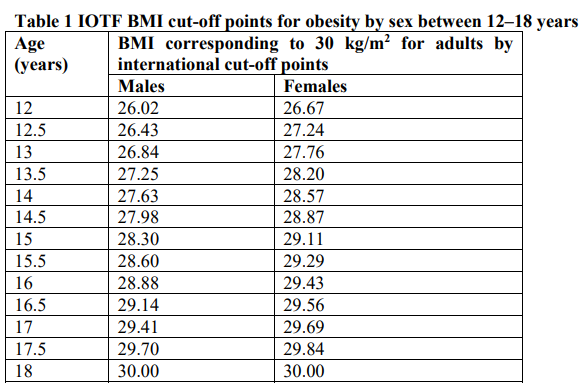
**4.3 Contraindications** Hypersensitivity to liraglutide or to any of the excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
A10BX07
xa 10 bx 07
Manufacturer Information
NOVO NORDISK PHARMA (SINGAPORE) PTE LTD
Novo Nordisk A/S
Novo Nordisk Pharmaceutical Industries, LP
Active Ingredients
Documents
Package Inserts
Saxenda Injection PI.pdf
Approved: November 14, 2022
