Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION
**Dosage and Administration** Treatment should be initiated under the supervision of a physician experienced in the management of haemophilia. _**Dosage:**_ The dosage and duration of the substitution therapy depend on the severity of Factor VIII deficiency, the location and the extent of the bleeding and on the patient’s clinical condition. Careful control of replacement therapy is especially important in cases of major surgery or life-threatening haemorrhages. The dose of Factor VIII administered is expressed in International Unit (IU), which is related to the WHO standard for Factor VIII products. Factor VIII activity in plasma is expressed either as a percentage (relative to normal human plasma) or in international units (relative to the international Standard for Factor VIII in plasma). One international unit of Factor VIII activity is equivalent to that quantity of Factor VIII in one mL of normal human plasma. The calculation of the required dosage of Factor VIII is based on the empirical finding that 1 international unit Factor VIII per kg body weight raises the plasma Factor VIII activity by 2 international units/dL. The dose is determined using the following formula and table.  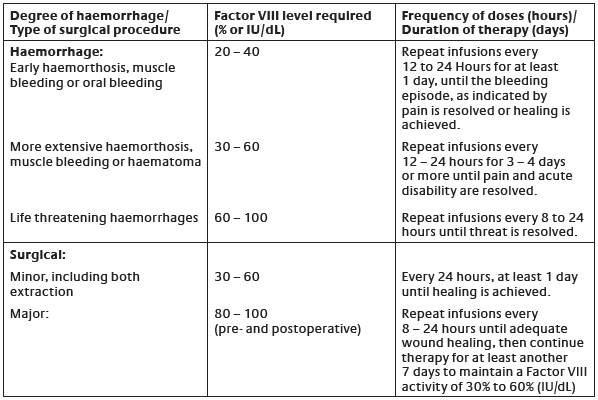 In case of the haemorrhagic events as shown in the table, the Factor VIII activity should not fall below the given plasma activity level (in % normal or international units/dL) in the corresponding period. The above table can be used to guide dosing in bleeding episodes and surgery. The amount and frequency of administration should be adapted to the clinical effectiveness of the product in the individual case. Under certain circumstances (e.g., presence of a low titre inhibitor) doses larger than those recommended may be necessary. During the course of treatment, appropriate determination of plasma Factor VIII levels is advised to guide the dose to be administered and the frequency of repeated infusions. If bleeding is not controlled with the recommended dose, the plasma level of Factor VIII should be determined and with a sufficient dose of ADVATE should be administered to achieve a satisfactory clinical response. In the case of major surgical interventions in particular, precise monitoring of the substitution therapy by means of plasma Factor VIII activity assay is indispensable. Individual patients may vary in their response to Factor VIII, achieving different levels of _in vivo_ recovery and demonstrating different half-lives. For long-term prophylaxis against bleeding in patients with severe haemophilia A, the usual doses are 20 to 40 international units of Factor VIII per kg body weight at intervals of 2 to 3 days. In some cases, especially in younger patients, shorter dose intervals or higher doses may be necessary. There are data on 13 pediatric patients collected on the use of ADVATE. _**Patients with inhibitors**_ Patients should be evaluated for the development of Factor VIII inhibitors, if the expected plasma Factor VIII activity levels are not attained, or if bleeding is not controlled with an appropriate dose. In patients with high levels of inhibitor, Factor VIII therapy may not be effective and other therapeutic options should be considered. Management of such patients should be directed by physicians with experience in the care of patients with haemophilia A. (see **Precautions**, under subheading **Inhibitor formation** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) _**Laboratory tests**_ Although dosage can be estimated by the calculations above, it is highly recommended that, whenever possible, appropriate laboratory tests including serial Factor VIII activity assays be performed. If the patient’s plasma Factor VIII fails to reach the expected levels or if bleeding is not controlled after adequate dosage, the presence of inhibitor should be suspected. By performing appropriate laboratory investigations, the presence of an inhibitor can be demonstrated and quantified in terms of international units Factor VIII neutralised by each mL of plasma. If the inhibitor is present at a level of less than 10 BU/mL, administration of additional Factor VIII may neutralise the inhibitor. Thereafter, the administration of additional Factor VIII should elicit the predicted response. The control of Factor VIII and inhibitor levels by laboratory assays is necessary in this situation. Inhibitor titres above 10 BU/mL may make haemostatic control with Factor VIII either impossible or impractical because of the large dose required. In addition, the inhibitor titre may rise following AHF infusion because of an anamnestic response to Factor VIII. _**Nature and contents of container**_ ADVATE powder and the solvent come in single-dose 5 mL vials of neutral borosilicate glass hydrolytic type I. The product vials are closed with Teflon coated butyl rubber stoppers and the solvent vials are closed with chlorobutyl rubber stoppers. Each vial is labelled for potency in international units, and is packaged together with 5 mL of sterilised water for injection, 1 BAXJECT II device for reconstitution, 1 mini-infusion set, 1 of 10 mL sterile disposable syringe for administration, 2 alcohol swabs and 2 plasters. _**Instructions for use, handling and disposal**_ The preparation is to be administered intravenously after reconstitution with the provided sterilised water for injection. Do not use after the expiry date printed on the label. Use within 3 hours after reconstitution. Do not refrigerate the preparation after reconstitution. Discard any unused preparation appropriately. _**Reconstitution using the BAXJECT II Device: Use Aseptic Technique**_ 1. Bring the ADVATE (dry factor concentrate) and Sterile Water for Injection (diluent) to room temperature. 2. Remove caps from the factor concentrate and diluent vials. 3. Cleanse stoppers with germicidal solution, and allow to dry prior to use. Place the vials on a flat surface. 4. Open the BAXJECT II device package by peeling away the lid, without touching the inside (Figure A). Do not remove the device from the package. 5. Turn the package over. Press straight down to fully insert the clear plastic spike through the diluent vial stopper (Figure B). 6. Grip the BAXJECT II package at its edge and pull the package off the device (Figure C). Do not remove the blue cap from the BAXJECT II device. Do not touch the exposed white plastic spike. 7. Turn the system over, so that the diluent vial is on top. Quickly insert the white plastic spike fully into the ADVATE vial stopper by pushing straight down (Figure D). The vacuum will draw the diluent into the ADVATE vial. 8. Swirl gently until ADVATE is completely dissolved. **NOTE: Do not refrigerate after reconstitution.** _**Administration: Use Aseptic Technique**_ Parenteral drug products should be inspected for particulate matter and discoloration prior to administration, whenever solution and container permit. A colourless appearance is acceptable for ADVATE. ADVATE should be administered at room temperature not more than 3 hours after reconstitution. Plastic syringes must be used with this product, since proteins such as ADVATE tend to stick to the surface of glass syringes. It is strongly recommended that every time ADVATE is administered, the patient name and batch number of the product are recorded to maintain a link between the patient and the batch of the product. 1. Remove the blue cap from the BAXJECT II device. Connect the syringe to the BAXJECT II device (Figure E). DO NOT INJECT AIR. 2. Turn the system upside down (factor concentrate vial now on top). Draw the factor concentrate into the syringe by pulling the plunger back slowly (Figure F). 3. Disconnect the syringe; attach a suitable needle and inject intravenously as instructed under **Administration by Bolus Infusion**. 4. If a patient is to receive more than one vial of ADVATE, the contents of multiple vials may be drawn into the same syringe. **Please note that the BAXJECT II reconstitution device is intended for use with a single vial of ADVATE and Sterile Water for Injection only, therefore reconstituting and withdrawing a second vial into the syringe requires a second BAXJECT II reconstitution device.** _**Administration by Bolus Infusion**_ A dose of ADVATE should be administered over a period of ≤ 5 minutes. The rate of administration should be a rate that ensures the comfort of the patient, up to a maximum of 10 mL/min. The pulse rate should be determined before and during administration of ADVATE. Should a significant increase in pulse rate occur, reducing the rate of administration or temporarily halting the injection usually allows the symptoms to disappear promptly. 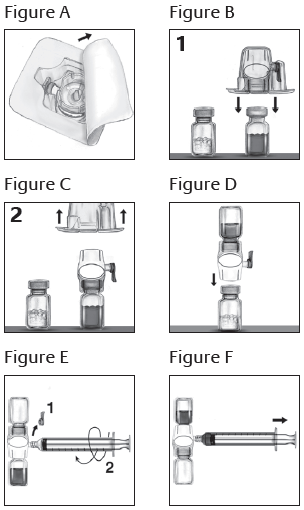 _**Administration by continuous infusion**_ The 1500, 1000 and 500 international units/vial nominal potency of ADVATE are suitable for use in a continuous infusion mode of administration. Continuous infusion of ADVATE must employ either a syringe pump running at a rate of greater than or equal to 0.4 mL/ hour, or a CADD-1 type infusion pump running at a rate of 1.5 mL/hour. _In vitro_ studies employing a syringe pump or CADD-1 pump have demonstrated > 80% of the hour 0 potency of ADVATE for up to 48 hours of continuous infusion. For sterility assurance purposes, a fresh supply of reconstituted ADVATE for continuous infusion (prepared under laminar air flow conditions) should be replaced at bedside no less frequently than every 12 hours. The post-reconstitution photostability of ADVATE is acceptable under the conditions of visible and ultra-violet light exposure in a clinical setting. It is highly recommended that Factor VIII levels be checked within 3 to 6 hours after the initiation of continuous infusion in order to document that the desired Factor VIII levels are being maintained. Rates of infusion should be modified based on the levels of plasma Factor VIII activity measured at least once per day thereafter and based on the desired level of Factor VIII. _**Overdosage**_ Of all infusions administered during clinical studies, 0.9% of infusions were >100 international units/kg. No safety concerns were identified with these infusions. No subject received a dose >208 international units/kg in these studies.
INTRAVENOUS
Medical Information
**Indications** ADVATE is indicated for use in haemophilia A for prevention and control of haemorrhagic episodes. Patients with haemophilia A may be treated with ADVATE as perioperative management. ADVATE is not indicated in von Willebrand’s disease.
**Contra-indications** Known hypersensitivity to any active substance, to excipients, or to mouse or hamster proteins.
B02BD02
coagulation factor VIII
Manufacturer Information
TAKEDA PHARMACEUTICALS (ASIA PACIFIC) PTE. LTD.
Takeda Manufacturing Austria AG
Baxalta Manufacturing Sarl
Siegfried Hameln GmbH (Solvent)
Active Ingredients
Documents
Package Inserts
Advate PI.pdf
Approved: May 25, 2022
