Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, POWDER, FOR SOLUTION
**4.2. DOSE AND METHOD OF ADMINISTRATION** BORTEZOMIB-AFT may be administered: - Intravenously (at a concentration of 1 mg/mL) as a 3 – 5 second bolus injection or - Subcutaneously (at a concentration of 2.5 mg/mL) Because each route of administration has a different reconstituted concentration, caution should be used when calculating the volume to be administered. At least 72 hours should elapse between consecutive doses of BORTEZOMIB-AFT. **BORTEZOMIB-AFT IS FOR INTRAVENOUS OR SUBCUTANEOUS USE ONLY. Intrathecal administration has resulted in death.** **Monotherapy** _**Relapsed/refractory multiple myeloma and relapsed/refractory mantle cell lymphoma (MCL)**_ Recommended dose The recommended dose of BORTEZOMIB-AFT SOLUTION FOR INJECTION 3.5 MG/VIAL is 1.3 mg/m2/dose administered twice weekly for two weeks (days 1, 4, 8, and 11) followed by a 10-day rest period (days 12 – 21). This 3-week period is considered a treatment cycle. At least 72 hours should elapse between consecutive doses of BORTEZOMIB-AFT. For extended therapy of more than 8 cycles, BORTEZOMIB-AFT may be administered on the standard schedule or on a maintenance schedule of once weekly for 4 weeks (days 1, 8, 15, and 22) followed by a 13-day rest period (days 23 – 35) (see **Section 5.1 Pharmacodynamic properties, Clinical trials** for a summary of dose administration during clinical trials – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Dose modification and re-initiation of therapy BORTEZOMIB-AFT therapy should be withheld at the onset of any Grade 3 non-haematological or Grade 4 haematological toxicities excluding neuropathy (see **Section 4.4 Special warnings and precautions for use** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Once the symptoms of the toxicity have resolved, BORTEZOMIB-AFT therapy may be re-initiated at a 25% reduced dose (1.3 mg/m2/dose reduced to 1.0 mg/m2/dose; 1.0 mg/m2/dose reduced to 0.7 mg/m2/dose). **Table 1** contains the recommended dose modification for the management of patients who experience BORTEZOMIB-AFT -related neuropathic pain and/or peripheral sensory neuropathy. Severe autonomic neuropathy resulting in treatment interruption or discontinuation has been reported. Patients with pre-existing severe neuropathy should be treated with BORTEZOMIB-AFT only after careful risk/benefit assessment. 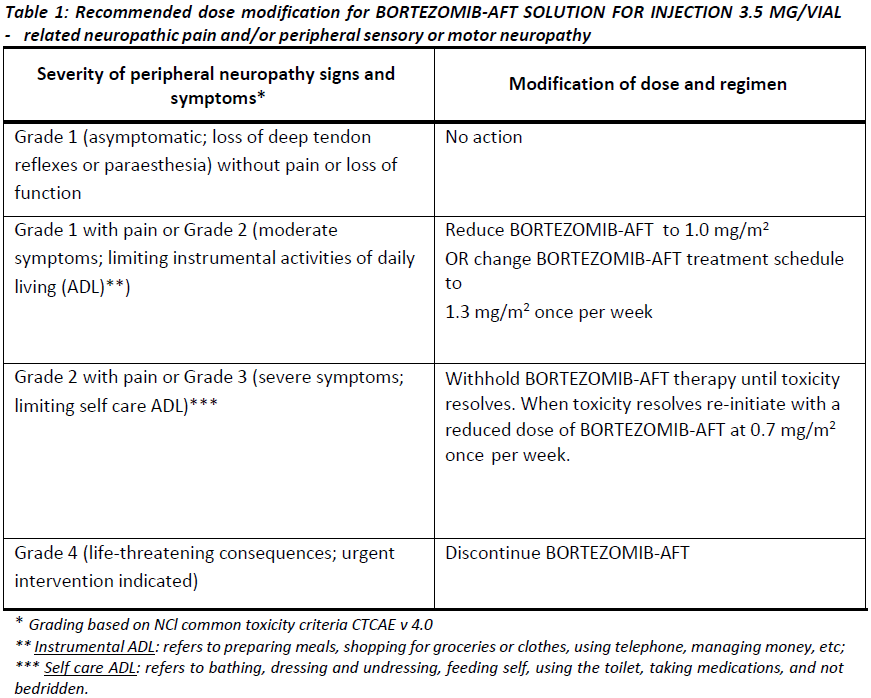 **Combination therapy** **_Previously untreated multiple myeloma – Non-transplant eligible_** Recommended dosage in combination with melphalan and prednisone BORTEZOMIB-AFT (bortezomib) for injection is administered in combination with oral melphalan and oral prednisone for nine 6-week treatment cycles as shown in **Table 2**. In cycles 1 – 4, BORTEZOMIB-AFT is administered twice weekly (days 1, 4, 8, 11, 22, 25, 29 and 32). In cycles 5 – 9, BORTEZOMIB-AFT is administered once weekly (days 1, 8, 22 and 29). At least 72 hours should elapse between consecutive doses of BORTEZOMIB-AFT. 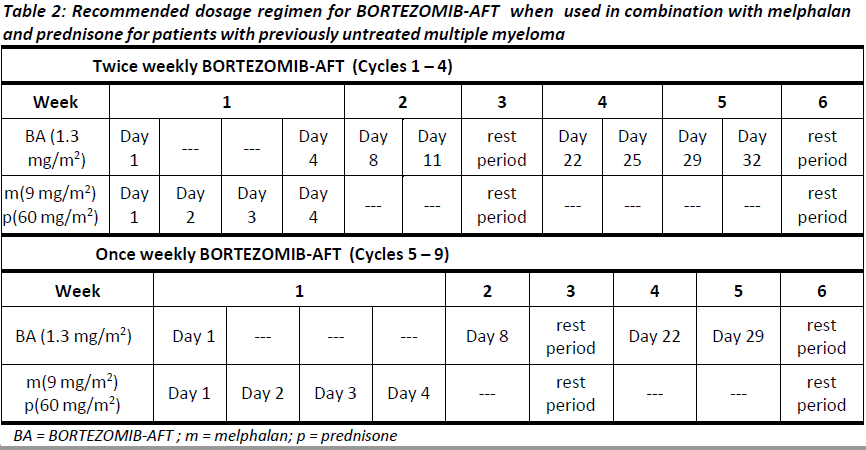 Dose management guidelines _Dose modification and re-initiation of therapy when BORTEZOMIB-AFT SOLUTION FOR INJECTION_ _3.5 MG/VIAL is administered in combination with melphalan and prednisone._ Prior to initiating a new cycle of therapy: - Platelet count should be ≥ 70 x 109/L and the ANC should be ≥ 1.0 x 109/L - Non-hematological toxicities should have resolved to Grade 1 or baseline 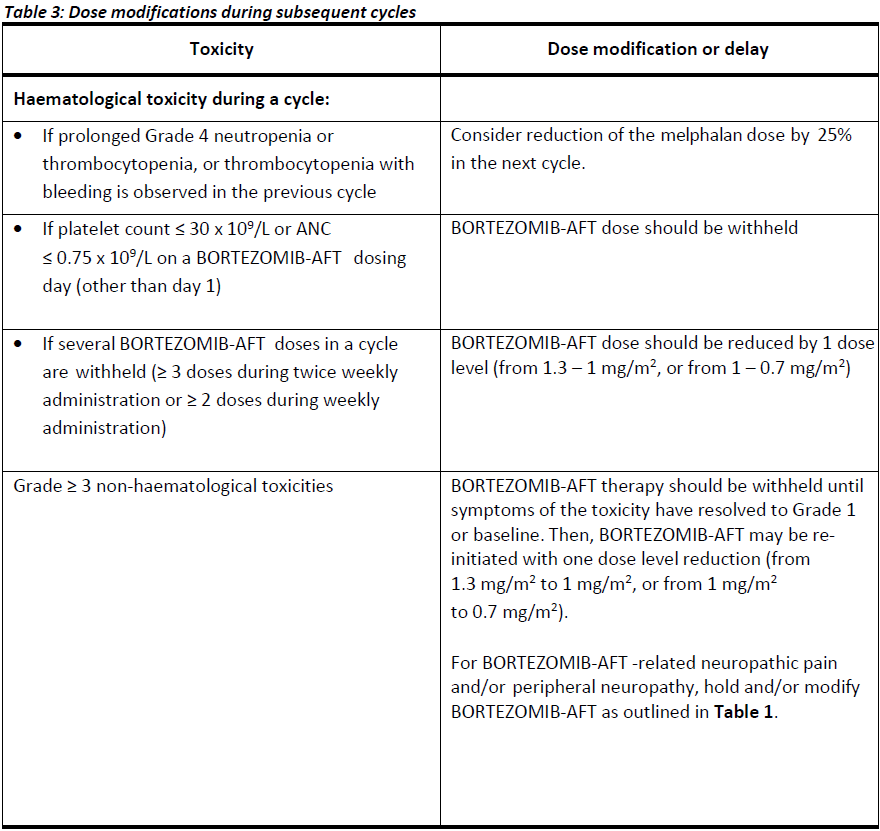 For additional information concerning melphalan and prednisone, see manufacturer’s Product Information documents. _**Previously untreated mantle cell lymphoma – Non-transplant eligible**_ Recommended dosage in combination with rituximab, cyclophosphamide, doxorubicin and prednisone BORTEZOMIB-AFT (bortezomib) for injection is administered at the recommended dose of 1.3 mg/m2 body surface area twice weekly for two weeks on days 1, 4, 8, and 11, followed by a 10-day rest period on days 12 – 21. This 3-week period is considered a treatment cycle. Six BORTEZOMIB-AFT cycles are recommended, although for patients with a response first documented at cycle 6, two additional BORTEZOMIB-AFT cycles may be given. At least 72 hours should elapse between consecutive doses of BORTEZOMIB-AFT. The following medicinal products are administered on Day 1 of each BORTEZOMIB-AFT 3-week treatment cycle as intravenous infusions: rituximab at 375 mg/m2, cyclophosphamide at 750 mg/m2, and doxorubicin at 50 mg/m2. Prednisone is administered orally at 100 mg/m2 on Days 1, 2, 3, 4 and 5 of each treatment cycle. Dose adjustments during treatment for patients with previously untreated mantle cell lymphoma Prior to initiating a new cycle of therapy (other than cycle 1): - Platelet count should be ≥ 100 x 109/L and absolute neutrophil count (ANC) should be ≥ 1.5 x 109/L - Haemoglobin should be ≥ 8 g/dL - Non-hematologic toxicity should have recovered to Grade 1 or baseline BORTEZOMIB-AFT treatment must be withheld at the onset of any ≥ Grade 3 BORTEZOMIB-AFT -related non haematological toxicities (excluding neuropathy) or ≥ Grade 3 haematological toxicities (see also **Section 4.4 Special warnings and precautions for use** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). For dose adjustments, see **Table 4** below. Colony stimulating factors may be administered for haematologic toxicity according to local standard practice. Platelet transfusion for the treatment of thrombocytopenia may be considered. 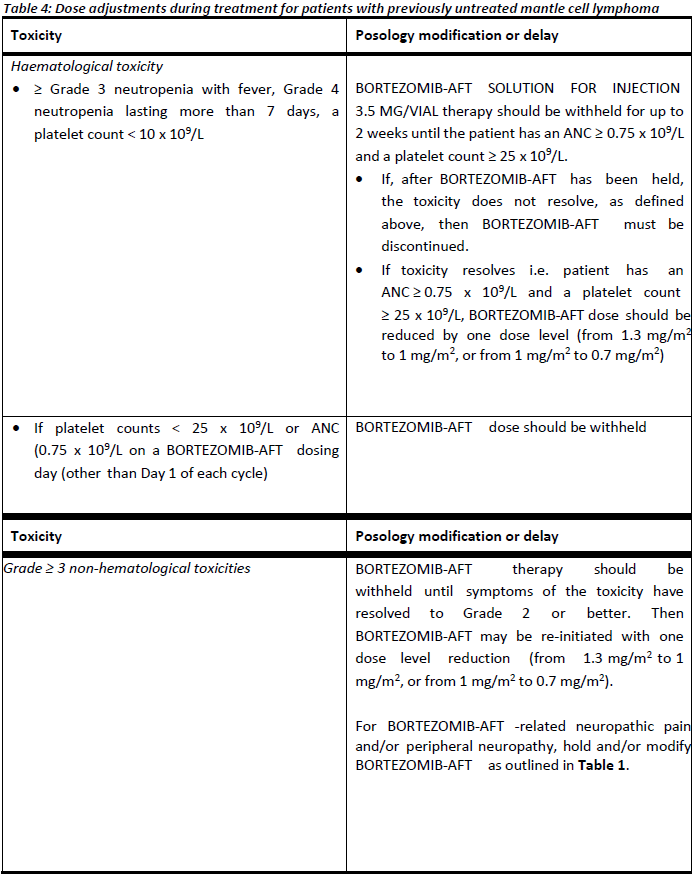 In addition, when BORTEZOMIB-AFT is given in combination with other chemotherapeutic medicinal products, appropriate dose reductions for these medicinal products should be considered in the event of toxicities, according to the recommendations in the respective Product Information documents. **Method of administration** **_Intravenous injection (IV)_** BORTEZOMIB-AFT is administered as a 3 – 5 second bolus intravenous injection through a peripheral or central intravenous catheter followed by a flush with 0.9% sodium chloride solution for injection. _**Subcutaneous injection (SC)**_ The reconstituted solution is injected into the thighs (right or left) or abdomen (right or left). Injection sites should be rotated for successive injections. If local injection site reactions occur following BORTEZOMIB-AFT injection subcutaneously, a less concentrated BORTEZOMIB-AFT solution (1 mg/mL instead of 2.5 mg/mL) may be administered subcutaneously or change to IV injection. When BORTEZOMIB-AFT is given in combination with other medicinal products, refer to the Product Information for these products for instructions for administration. **Instructions for use and handling and disposal** _**Administration precautions:**_ BORTEZOMIB-AFT is an antineoplastic. Caution should be used during handling and preparation, including careful dose calculation to prevent overdose. The drug quantity in one vial (3.5 mg) may exceed the usual single dose required. Proper aseptic technique should be used. Use of gloves and other protective clothing to prevent skin contact is recommended. In clinical trials, local skin irritation was reported in 5% of patients, but extravasation of bortezomib was not associated with tissue damage. When administered subcutaneously, alternate sites for each injection (thigh or abdomen). New injections should be given at least one inch from an old site and never into areas where the site is tender, bruised, red, or hard. There have been fatal cases of inadvertent intrathecal administration of bortezomib. BORTEZOMIB-AFT is for IV and subcutaneous use only. **DO NOT ADMINISTER BORTEZOMIB-AFT INTRATHECALLY**. _**Reconstitution/Preparation for administration for intravenous and subcutaneous administration:**_ Prior to use, the contents of each vial must be reconstituted only with normal (0.9%) saline (sodium chloride for injection) according to the following instructions based on route of administration: The reconstituted product should be a clear and colourless solution. Parenteral drug products should be inspected visually for particulate matter and discolouration prior to administration whenever solution and container permit. If any discolouration or particulate matter is observed, the reconstituted product should not be used. _**Procedure for proper disposal:**_ Discard any residue. Any unused product or waste material should be disposed of in accordance with local requirements. **Dosage adjustment in:** **_Patients with Renal Impairment_** The pharmacokinetics of bortezomib are not influenced by the degree of renal impairment. Therefore, dosing adjustments of bortezomib are not necessary for patients with renal insufficiency. Since dialysis may reduce bortezomib concentrations, the drug should be administered after the dialysis procedure (see section 5.2 Pharmacokinetic Properties – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _**Hepatic insufficiency**_ Patients with mild hepatic impairment do not require a starting dose adjustment and should be treated per the recommended BORTEZOMIB-AFT dose. Patients with moderate or severe hepatic impairment should be started on BORTEZOMIB-AFT at a reduced dose of 0.7 mg/m2 per injection during the first cycle, and a subsequent dose escalation to 1.0 mg/m2 or further dose reduction to 0.5 mg/m2 may be considered based on patient tolerance (see **Table 5**). 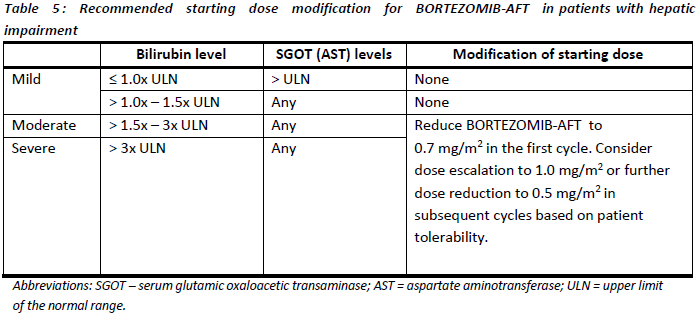
INTRAVENOUS, SUBCUTANEOUS
Medical Information
**4.1. THERAPEUTIC INDICATIONS** BORTEZOMIB-AFT is indicated as part of combination therapy for the treatment of patients with previously untreated multiple myeloma. BORTEZOMIB-AFT is indicated as monotherapy for the treatment of patients with multiple myeloma who have received at least 1 prior therapy. BORTEZOMIB-AFT is indicated as monotherapy for the treatment of patients with mantle cell lymphoma who have received at least 1 prior therapy. BORTEZOMIB-AFT in combination with rituximab, cyclophosphamide, doxorubicin and prednisone is indicated for the treatment of adult patients with previously untreated mantle cell lymphoma who are unsuitable for haematopoietic stem cell transplantation.
**4.3. CONTRAINDICATIONS** BORTEZOMIB-AFT is contraindicated in patients with hypersensitivity to bortezomib, boron or mannitol. BORTEZOMIB-AFT is also contraindicated in patients with acute diffuse infiltrative pulmonary and pericardial disease.
L01XG01
bortezomib
Manufacturer Information
APEX PHARMA MARKETING PTE. LTD.
QILU PHARMACEUTICAL (HAINAN) CO LTD
Active Ingredients
Documents
Package Inserts
Bortezomib-AFT Powder For Solution For Injection 3.5mg (vial) PI.pdf
Approved: March 22, 2023
