Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, SOLUTION
**5.2 Posology and method of administration** Routes of administration Fentanyl should be given only in an environment where the airway can be controlled and by personnel who can control the airway (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Intravenous administration, either as a bolus or by infusion. Intramuscular administration. To avoid bradycardia, it is recommended to administer a small intravenous dose of an anti-cholinergic just before anaesthetic induction. Posology Fentanyl Injection 50 micrograms/ml, by the intravenous route, can be administered to both adults and children. The dose of Fentanyl Injection 50 micrograms/ml should be individualised according to age, body weight, physical status, underlying pathological condition, use of other drugs and type of surgery and anaesthesia. Adults The usual dosage regime is as follows: 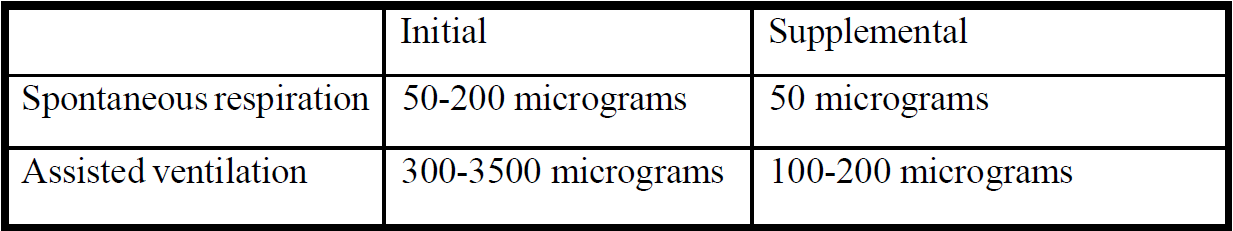 Doses in excess of 200 micrograms are for use in anaesthesia only. As a premedicant, 1–2 ml Fentanyl Injection 50 micrograms/ml may be administered intramuscularly 45 minutes before induction of anaesthesia. After intravenous administration in unpremedicated adult patients, 2 ml of Fentanyl Injection 50 micrograms/ml may be expected to provide sufficient analgesia for 10 – 20 minutes in surgical procedures involving low pain intensity. 10 ml Fentanyl Injection 50 micrograms/ml injected as a bolus gives analgesia lasting about one hour. The analgesia produced is sufficient for surgery involving moderately painful procedures. Giving a dose of 50mcg/kg Fentanyl Injection 50 micrograms/ml will provide intense analgesia for some four to six hours for intensely stimulating surgery. Fentanyl Injection 50 micrograms/ml may also be given as an infusion. In ventilated patients, a loading dose of Fentanyl Injection 50 micrograms/ml may be given as a fast infusion of approximately 1 mcg/kg/min for the first 10 minutes followed by an infusion of approximately 0.1 mcg/kg/min. Alternatively, the loading dose of Fentanyl Injection 50 micrograms/ml may be given as a bolus. Infusion rates should be titrated to individual patient response; lower infusion rates may be adequate. Unless it is planned to ventilate post-operatively, the infusion should be terminated at about 40 minutes before the end of surgery. Lower infusion rates, e.g. 0.05–0.08 mcg/kg/minute are necessary if spontaneous ventilation is to be maintained. Higher infusion rates (up to 3 mcg/kg/minute) have been used in cardiac surgery. Fentanyl Injection is chemically incompatible with the induction agents thiopentone and methohexitone because of wide differences in pH. _Use in elderly and debilitated patients:_ It is wise to reduce the dosage in the elderly and debilitated patients. The effect of initial dose should be taken into account in determining supplemental doses. _Paediatric population_ The doctor calculates the best dosage based on the child’s weight, severity of pain and length of pain relief required. The initial dose being 3 – 5mcg per kilogram body weight followed as required by 1mcg per kilogram body weight. If breathing is artificially assisted, the initial dose can be increased up to a maximum of 15mcg per kilogram body weight, followed as required by 1–3mcg per kilogram body weight. _Use in children:_ Analgesia during operation, enhancement of anaesthesia with spontaneous respiration. Techniques that involve analgesia in a spontaneous breathing child should only be used as part of an anaesthetic technique, or given as part of a sedation/analgesia technique with experienced personnel in an environment that can manage sudden chest wall rigidity requiring intubation, or apnoea requiring airway support (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). It is important when estimating the required dose to assess the likely degree of surgical stimulation, the effect of premedicant drugs, and the duration of the procedure. _Obese patients:_ In obese patients there is a risk of overdosing if the dose is calculated based on body weight. Obese patients should have dosage calculated according to their estimated ideal body mass. _Renal Impairment_ In patients with renal impairment reduced dosing of fentanyl should be considered and these patients should be observed carefully for signs of fentanyl toxicity (see section 5.2 Pharmacokinetic properties – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
INTRAVENOUS, INTRAMUSCULAR
Medical Information
**5.1 Therapeutic indications** Fentanyl citrate is an opioid analgesic used: In low doses to provide analgesia during short surgical procedures. In high doses as an analgesic/respiratory depressant in patients requiring assisted ventilation. In combination with a neuroleptic in the technique of neuroleptanalgesia.
**5.3 Contraindications** Hypersensitivity to the Fentanyl citrate or to any of the excipients listed in section 6.1 Respiratory depression, obstructive airways disease – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. Concurrent administration with monoamine oxidase inhibitors, or within 2 weeks of their discontinuation. Known intolerance to fentanyl citrate or other morphinomimetics.
N01AH01
fentanyl
Manufacturer Information
ALLIANCE PHARM PTE. LTD.
MACARTHYS LABORATORIES LIMITED T\A MARTINDALE PHARMA
Active Ingredients
Documents
Package Inserts
Fentanyl Soln for Injection 50mcg per ml package insert.pdf
Approved: May 16, 2019
