Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, POWDER, LYOPHILIZED, FOR SUSPENSION
**Posology and Method of Administration** The recommended dose of DORIBAX™ is 500 mg administered every 8 hours by intravenous infusion. The recommended dosage and administration by infection is described in Table 1: 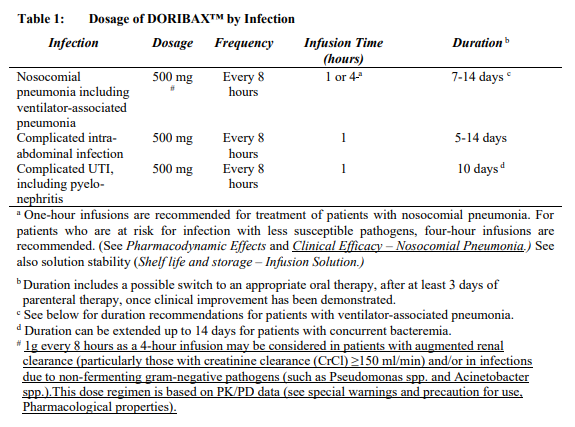 The usual treatment duration for patients with nosocomial pneumonia, including ventilator-associated pneumonia, is 7 to 14 days and is often in the upper range for patients infected with non-fermenting gram-negative pathogens (such as _Pseudomonas_ spp. and _Acinetobacter_ spp.). Treatment should be guided by the severity of illness, infecting pathogen and the patient’s clinical response. In a Phase 3 study in patients with ventilator-associated pneumonia, a 7-day course of DORIBAX™ (1 gram every 8 hours as a 4 hour infusion) has been associated with a higher mortality rate and a lower clinical cure rate compared to a 10-day course of imipenem/cilastatin therapy. Consideration should be given to treat patients with ventilator-associated pneumonia for more than 7 days \[see _Undesirable Effects_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_\]. **Patients with Renal Impairment** In patients whose creatinine clearance (CrCl) is > 50 ml/min, no dosage adjustment is necessary. In patients with moderate renal impairment (CrCl ≥ 30 to ≤ 50 ml/min), the dosage of DORIBAX™ should be 250 mg administered every 8 hours. In patients with severe renal impairment (CrCl > 10 to < 30 ml/min), the dosage of DORIBAX™ should be 250 mg administered every 12 hours. (See _Preparation of 500 mg dose of DORIBAX™ solution for infusion and Preparation of 250 mg dose of DORIBAX™ solution for infusion for patients with moderate or severe renal impairment_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Due to limited clinical data and an expected increased exposure to doripenem, DORIBAX™ should be used with caution in patients with severe renal impairment (see _Pharmacokinetic Properties_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The following formula may be used to estimate CrCl. The serum creatinine used in the formula should represent a steady state of renal function. 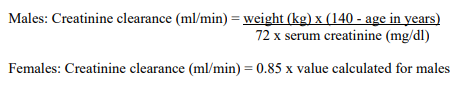 **Patients on Dialysis** DORIBAX™ dosing and administration recommendations for patients on continuous renal replacement therapies are shown in Table 2: 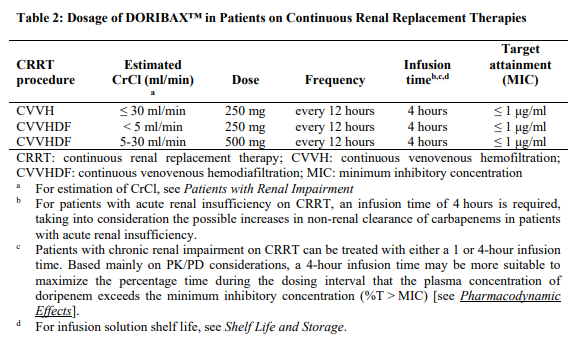 Dosing recommendations for pathogens with MIC >1 mcg/ml have not been established for continuous renal replacement therapy due to the potential for accumulation of doripenem and doripenem-M-1 metabolite \[see _Continuous Renal Replacement Therapy and Patients on Dialysis_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_\]. Close safety monitoring is advised for patients on continuous renal replacement therapy, due to limited clinical data and an expected increased exposure to doripenem-M-1 metabolite \[see _Continuous Renal Replacement Therapy_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_\]. There is insufficient information to make dose adjustment recommendations for patients on other forms of dialysis \[see _Patients on Dialysis_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_\]. **Patients with Hepatic Impairment** No dosage adjustment is necessary. **Age, Gender and Race** No dosage adjustment is recommended based on age (18 years of age and older) gender or race.
INTRAVENOUS
Medical Information
**Therapeutic Indications** DORIBAX™ is a carbapenem antibiotic indicated as a single agent for the treatment of the following infections caused by susceptible bacteria: (See _Microbiology_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.) - Complicated intra-abdominal infections - Complicated urinary tract infections, including complicated and uncomplicated pyelonephritis and cases with concurrent bacteremia Doribax is also indicated for the treatment of nosocomial pneumonia, including ventilator-associated pneumonia, caused by susceptible bacteria. In the nosocomial pneumonia clinical studies, adjunctive use of an aminoglycoside was permitted. Because of its broad spectrum of bactericidal activity against gram-positive and gram-negative aerobic and anaerobic bacteria, DORIBAX™ can be considered for treatment of complicated and mixed infections. Appropriate specimens for bacteriological examination should be obtained in order to isolate and identify causative organisms and to determine their susceptibility to doripenem. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
**Contraindications** DORIBAX™ is contraindicated in patients with known serious hypersensitivity to doripenem or to other drugs in the same class or in patients who have demonstrated anaphylactic reactions to beta-lactams.
Manufacturer Information
CELLTRION HEALTHCARE SINGAPORE PRIVATE LIMITED
Shionogi Pharma Co., Ltd.
Active Ingredients
Documents
Package Inserts
Doribax Powder for Injection PI.pdf
Approved: June 7, 2021
