Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**4.2 Dosage and method of administration** _Prevention of VTE in patients undergoing total hip replacement or total knee replacement surgery_ The recommended dose is 10 mg rivaroxaban taken orally once daily. The initial dose should be taken within 6 to 10 hours after surgery, provided that haemostasis has been established. The duration of treatment depends on the individual risk of the patient for venous thromboembolism which is determined by the type of orthopaedic surgery. - For patients undergoing major hip surgery, a treatment duration of 5 weeks is recommended. - For patients undergoing major knee surgery, a treatment duration of 2 weeks is recommended. If a dose is missed the patient should take the 10mg Xarelto dose immediately and then continue on the following day with the once daily intake as before. _Treatment of DVT, treatment of PE and prevention of recurrent DVT and PE_ The recommended dose for the initial treatment of acute DVT or PE is 15 mg twice daily for the first three weeks followed by 20 mg once daily for the continued treatment and prevention of recurrent DVT and PE. Short duration of therapy (at least 3 months) should be considered in patients with DVT or PE provoked by major transient risk factors (i.e. recent major surgery or trauma). Longer duration of therapy should be considered in patients with provoked DVT or PE not related to major transient risk factors, unprovoked DVT or PE, or a history of recurrent DVT or PE. When extended prevention of recurrent DVT and PE is indicated (following completion of at least 6 months therapy for DVT or PE), the recommended dose is 10 mg once daily. In patients in whom the risk of recurrent DVT or PE is considered high, such as those with complicated comorbidities, or who have developed recurrent DVT or PE on extended prevention with Xarelto 10 mg once daily, a dose of Xarelto 20 mg once daily should be considered. The duration of therapy and dose selection should be individualised after careful assessment of the treatment benefit against the risk for bleeding (see section ‘Special warnings and precautions for use’ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). 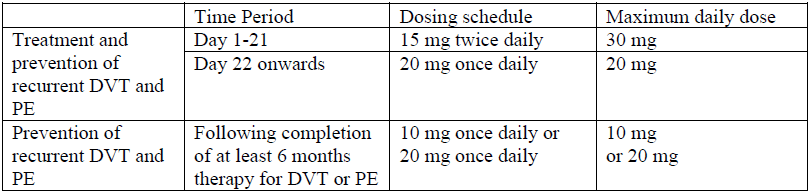 It is essential to adhere to the dosage schedule provided. If a dose is missed during the 15 mg twice daily treatment phase, the patient should take Xarelto immediately to ensure intake of 30 mg Xarelto per day. In this case two 15 mg tablets may be taken at once. The patient should continue with the regular 15 mg twice daily intake as recommended on the following day. If a dose is missed during the once daily treatment phase, the patient should take Xarelto immediately to ensure intake of the recommended daily dose. The patient should continue with the regular once daily dose intake as recommended on the following day. Intake of Xarelto in relation to food Xarelto may be taken with or without food. For patients who are unable to swallow whole tablets, Xarelto tablet may be crushed and mixed with water or soft foods such as applesauce immediately prior to use and administered orally. The crushed Xarelto tablet may be given through gastric tubes. Gastric placement of the tube should be confirmed before administering Xarelto. The crushed tablet should be administered in a small amount of water via a gastric tube after which it should be flushed with water. (see section ‘Pharmacokinetic properties’ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Additional information on special populations** _Children and adolescents_ Safety and efficacy have not been established in children and adolescents below 18 years. Xarelto is not recommended for use in children or adolescents below 18 years of age due to a lack of data on safety and efficacy. _Geriatric patients_ No dose adjustment is required based on age. _Gender_ No dose adjustment is required based on gender. _Body weight_ No dose adjustment is required based on body weight. _Patients with hepatic impairment_ Xarelto is contraindicated in patients with hepatic disease which is associated with coagulopathy leading to a clinically relevant bleeding risk (see sections ‘Contraindications’ and ‘Pharmacokinetic properties’ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Xarelto may be used with caution in cirrhotic patients with moderate hepatic impairment (Child Pugh B) if it is not associated with coagulopathy (see sections ‘Special warnings and precautions for use’ and ‘Pharmacokinetic properties’ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). No dose adjustment is necessary in patients with other hepatic diseases. _Patients with renal impairment_ Limited clinical data for patients with severe renal impairment (creatinine clearance 15 – 29 ml/min) indicate that rivaroxaban plasma concentrations are significantly increased. Therefore, Xarelto is to be used with caution in these patients. Use is not recommended in patients with creatinine clearance < 15 ml/min (see sections ‘Special warnings and precautions for use’ and ‘Pharmacokinetic properties’ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). - For the prevention of VTE in adult patients undergoing elective hip or knee replacement surgery, no dose adjustment is necessary in patients with mild renal impairment (creatinine clearance 50 – 80 ml/min) or moderate renal impairment (creatinine clearance 30 – 49 ml/min) (see section ‘Pharmacokinetic properties’ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). - For the treatment of DVT, treatment of PE and prevention of recurrent DVT and PE, no dose adjustment from the recommended dose is necessary in patients with mild renal impairment (creatinine clearance 50 – 80 ml/min) (see section ‘Pharmacokinetic properties’ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). In patients with moderate (creatinine clearance 30 – 49 ml/min) or severe (creatinine clearance 15 – 29 ml/min) renal impairment: patients should be treated with 15 mg twice daily for the first 3 weeks. Thereafter, when the recommended dose is 20 mg once daily, a reduction of the dose from 20 mg once daily to 15 mg once daily should be considered if the patient’s assessed risk for bleeding outweighs the risk for recurrent DVT and PE. The recommendation for the use of 15 mg is based on PK modelling and has not been studied in this clinical setting (see sections ‘Special warnings and precautions for use’, ‘Pharmacodynamic properties’ and ‘Pharmacokinetic properties’ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). When the recommended dose is 10 mg once daily, no dose adjustment from the recommended dose is necessary. **Converting from Vitamin K Antagonist (VKA) to Xarelto** For patients treated for DVT, PE and prevention of recurrence, VKA treatment should be stopped and Xarelto therapy should be initiated once the INR is ≤2.5. When converting patients from VKAs to Xarelto, INR values will be falsely elevated after the intake of Xarelto. The INR is not valid to measure the anticoagulant activity of Xarelto, and therefore should not be used (see section ‘Interaction with other medicinal products and other forms of interaction’ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Converting from Xarelto to Vitamin K antagonists (VKA)** There is a potential for inadequate anticoagulation during the transition from Xarelto to VKA. Continuous adequate anticoagulation should be ensured during any transition to an alternate anticoagulant. It should be noted that Xarelto can contribute to an elevated INR. In patients converting from Xarelto to VKA, VKA should be given concurrently until the INR is ≥2.0. For the first two days of the conversion period, standard VKA dosing should be used followed by VKA dosing guided by INR testing. While patients are on both Xarelto and VKA, the INR should not be tested earlier than 24 hours (after the previous dose but prior to the next dose of Xarelto. Once Xarelto is discontinued INR testing may be done reliably 24 hours after the last dose (see section ‘Interaction with other medicinal products and other forms of interaction’ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Converting from parenteral anti-coagulants to Xarelto** For patients currently receiving a parenteral anticoagulant, start Xarelto 0 to 2 hours before the time of the next scheduled administration of the parenteral drug (e.g. LMWH) or at the time of discontinuation of a continuously administered parenteral drug (e.g. intravenous unfractionated heparin). **Converting from Xarelto to parenteral anti-coagulants** Discontinue Xarelto and give the first dose of parenteral anticoagulant at the time that the next Xarelto dose would be taken.
ORAL
Medical Information
**4.1 Indications** Prevention of venous thromboembolism (VTE) in patients undergoing total hip replacement or total knee replacement surgery. Xarelto is indicated for the treatment of Deep Vein Thrombosis (DVT) and pulmonary embolism (PE), and for the prevention of recurrent DVT, PE in adults. (See section ‘Special warnings and precautions for use’ for haemodynamically unstable PE patients – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.)
**4.3 Contraindications** Hypersensitivity to rivaroxaban or any excipient of the tablet. Clinically significant active bleeding (e.g.,intracranial bleeding, gastrointestinal bleeding). Hepatic disease which is associated with coagulopathy leading to a clinically relevant bleeding risk (see section ‘Pharmacokinetic properties’ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Pregnancy and lactation (see section ‘Pregnancy and lactation’ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Lesion or condition if considered to be a significant risk of major bleeding .This may include current or recent gastrointestinal ulceration, presence of malignant neoplasms at high risk of bleeding, recent brain or spinal injury, recent brain, spinal or ophthalmic surgery, recent intracranial haemorrhage, known or suspected oesophageal varices, arteriovenous malformations, vascular aneurysms or major intraspinal or intracerebral vascular abnormalities. Concomitant treatment with any other anticoagulant agent e.g. unfractionated heparin (UFH), low molecular weight heparins (enoxaparin, dalteparin, etc.), heparin derivatives (fondaparinux, etc.), oral anticoagulants (warfarin, apixaban, dabigatran, etc.) except under the circumstances of switching therapy to or from rivaroxaban (see section ‘Dosage and method of administration’) or when UFH is given at doses necessary to maintain an open central venous or arterial catheter. (see section ‘Interaction with other medicinal products and other forms of interaction’ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_)
B01AF01
rivaroxaban
Manufacturer Information
BAYER (SOUTH EAST ASIA) PTE LTD
Bayer AG
Bayer HealthCare Manufacturing S.r.l.
Bayer Bitterfeld GmbH
Active Ingredients
Documents
Package Inserts
Xarelto Film-Coated Tablet 10mg PI.pdf
Approved: February 16, 2023
