Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, SOLUTION
**4.2 Posology and method of administration** Posology The recommended initial dose of etelcalcetide is 5 mg administered by bolus injection 3 times per week. Corrected serum calcium should be at or above the lower limit of the normal range prior to administration of first dose of Parsabiv, a dose increase, or reinitiation after a dose stop (see also dose adjustments based on serum calcium levels). Parsabiv should not be administered more frequently than 3 times per week. _Dose titration_ Parsabiv should be titrated so that doses are individualised between 2.5 mg and 15 mg. The dose may be increased in 2.5 mg or 5 mg increments no more frequently than every 4 weeks to a maximum dose of 15 mg 3 times per week to achieve the desired parathyroid hormone (PTH) target. _Dose adjustments based on PTH levels_ PTH should be measured after 4 weeks from initiation or dose adjustment of Parsabiv, and approximately every 1–3 months during maintenance. Dose adjustment may be necessary at any time during treatment including the maintenance phase. If PTH is below 100 pg/mL (10.6 pmol/L), the dose should be reduced or temporarily stopped. If PTH does not return to > 100 pg/mL following dose reduction, the dose should be stopped. For patients in whom the dose is stopped, Parsabiv should be reinitiated at a lower dose once PTH returns to > 150 pg/mL (15.9 pmol/L) and pre-dialysis serum corrected calcium (cCa) ≥ 8.3 mg/dL (2.08 mmol/L). If the patient’s last administered dose was 2.5 mg, Parsabiv may be reinitiated at the 2.5 mg dose level if PTH is > 300 pg/mL (31.8 pmol/L), and the most recent pre-dialysis serum cCa ≥ 8.3 mg/dL (2.08 mmol/L). Additional recommendations related to the management of low calcium are provided in the table below. Parsabiv may be used as part of a therapeutic regimen including phosphate binders and/or vitamin D sterols, as appropriate (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Dose adjustments based on serum calcium levels_ Serum calcium should be measured within 1-week of initiation or dose adjustment of Parsabiv. Once the maintenance phase has been established for a patient, corrected serum calcium should be measured approximately every 4 weeks. In the studies total serum calcium was measured using Roche modular analysers. The lower limit of the normal range for corrected serum calcium was 8.3 mg/dL (2.08 mmol/L). Other laboratory assays may have different cut-offs for the lower limit of the normal range. In the event that clinically meaningful decreases in corrected serum calcium levels below the lower limit of the normal range occur and/or symptoms of hypocalcaemia occur, the following management is recommended: 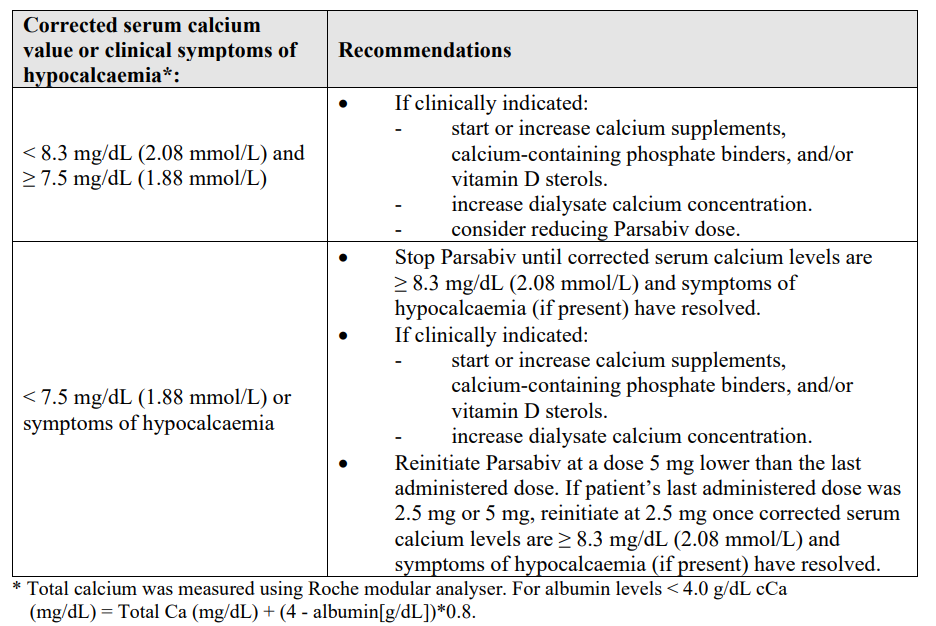 _Switch from cinacalcet to etelcalcetide_ Etelcalcetide should not be initiated in patients until 7 days after the last dose of cinacalcet and the corrected serum calcium is at or above the lower limit of the normal range (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Missed doses_ If a regularly scheduled haemodialysis treatment is missed, do not administer any missed doses. Parsabiv should be administered at the next haemodialysis treatment at the same dose. If doses are missed for more than 2 weeks, then Parsabiv should be administered at 5 mg, (or 2.5 mg if that was the patients last administered dose), and titrated to achieve the desired PTH. _Special population_ _Elderly_ Dosing recommendations for elderly patients are the same as for adult patients. _Paediatric population_ The safety and efficacy of etelcalcetide in children and adolescents less than 18 years have not yet been established. No data are available. Method of administration Parsabiv is administered into the venous line of the dialysis circuit at the end of the haemodialysis treatment during rinse-back or intravenously after rinse-back. When given during rinse-back at least 150 mL of rinse-back volume should be administered after injection. If rinse-back is completed and Parsabiv was not administered, then it may be administered intravenously followed by at least 10 mL sodium chloride 9 mg/mL (0.9%) solution for injection flush volume. Parsabiv should not be diluted. Parenteral medicinal products should be inspected visually for particulate matter and change in colour prior to administration.
INTRAVENOUS
Medical Information
**4.1 Therapeutic indications** Parsabiv is indicated for the treatment of secondary hyperparathyroidism (SHPT) in adult patients with chronic kidney disease (CKD) on haemodialysis therapy.
**4.3 Contraindications** Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. Parsabiv should not be initiated if corrected serum calcium is less than the lower limit of the normal range (see sections 4.2 and 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
H05BX04
etelcalcetide
Manufacturer Information
AMGEN BIOTECHNOLOGY SINGAPORE PTE. LTD.
Patheon Manufacturing Services LLC (Bulk Production and Primary Packager)
Amgen Manufacturing Limited
Active Ingredients
Documents
Package Inserts
PARSABIV SOLUTION FOR INJECTION PI.pdf
Approved: April 11, 2022
