Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
SYRUP
**Posology** Dosage and duration of therapy are dependent upon the extent of iron deficiency. Iron deficiency anaemia: the therapy takes about 3–5 months until a normalisation of the haemoglobin value is achieved. Afterwards the therapy should be continued for several weeks, or for pregnant women, at least until the end of the pregnancy with a dosage such as described for iron deficiency without anaemia in order to replenish the iron stores. Iron deficiency without anaemia: the therapy takes about 1–2 months. 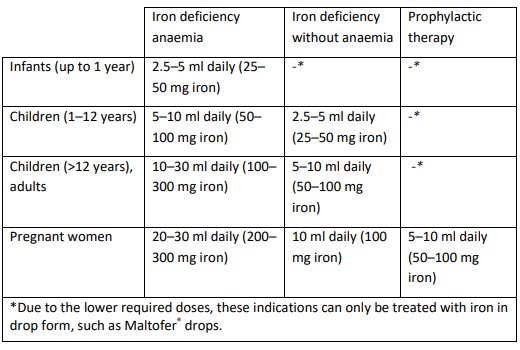 **Method of administration** The daily dosage can be divided into separate doses or can be taken all at one time. Maltofer® syrup should be taken during or immediately after a meal. Maltofer® syrup can be mixed with fruit and vegetable juices or with bottle-feed. The slight discolouration of the mixture does not affect either the efficacy of the product nor the taste of the drink to which it is added. The supplied measuring cup is used for an exact administration of the dosage.
ORAL
Medical Information
**Therapeutic indications** Treatment of iron deficiency without anaemia and iron deficiency anaemia. Prophylactic therapy of iron deficiency during pregnancy.
**Contraindications** - Known hypersensitivity or intolerance to iron(III)-hydroxide polymaltose complex or any of the excipients - Iron overload (e.g. haemochromatosis, haemosiderosis) - Disturbances in iron utilisation (e.g. anaemia from lead-poisoning, sidero-achrestic anaemia, thalassaemia) - Anaemia not caused by iron deficiency (e.g. haemolytic anaemia or megaloblastic anaemia due to vitamin B12 deficiency).
B03AB05
ferric oxide polymaltose complexes
Manufacturer Information
VIFOR PHARMA ASIA PACIFIC PTE. LTD.
Vifor SA
Active Ingredients
Documents
Package Inserts
Maltofer Syrup PI.pdf
Approved: September 21, 2020
