Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, POWDER, FOR SOLUTION
**2.2. DOSAGE AND ADMINISTRATION** **General** Cymevene must be reconstituted and diluted under the supervision of a healthcare professional and administered as an intravenous infusion (see section 4.2 Special Instructions for Use, Handling and Disposal – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Caution: Cymevene must only be administered by IV infusion over 1 hour, preferably via a plastic cannula, into a vein with adequate blood flow (intramuscular or subcutaneous injection may result in severe tissue irritation due to the high pH (~ 11) of ganciclovir solution). Do not administer by rapid or bolus IV injection because the resulting excessive plasma levels may increase the toxicity of Cymevene (see section 4.2 Special Instructions for Use, Handling and Disposal – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The recommended dosage, frequency, or infusion rates should not be exceeded. **STANDARD DOSAGE FOR TREATMENT OF CMV DISEASE** Dosage for patients with normal renal function _Induction treatment:_ 5 mg/kg given as an IV infusion over one hour, every 12 hours for 14–21 days. _Maintenance treatment:_ For immunocompromised patients at risk of relapse maintenance therapy may be given. 5 mg/kg given as an IV infusion over one hour, once daily on 7 days per week or 6 mg/kg once daily on 5 days per week. The duration of maintenance treatment should be determined on an individual basis. **STANDARD DOSAGE FOR PREVENTION OF CMV DISEASE USING PRE-EMPTIVE THERAPY** Dosage for patients with normal renal function _Induction treatment:_ 5 mg/kg given as an IV infusion over one hour, every 12 hours for 7–14 days. _Maintenance treatment:_ 5 mg/kg given as an IV infusion over one hour, once daily on 7 days per week or 6 mg/kg once daily on 5 days per week. The duration of maintenance treatment is based on the risk of CMV disease and should be determined on an individual basis. **2.2.1. Special dosage instructions** _Pediatric Patients_ Safety and efficacy of ganciclovir in pediatrics have not been established, including use for the treatment of congenital or neonatal CMV infections. The use of Cymevene in children warrants extreme caution due to the potential for long-term carcinogenicity and reproductive toxicity. The benefits of treatment should outweigh the risks (see section 3.2.5 Pharmacokinetics in Special Populations, Pediatric Population – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Geriatric patients_ No studies have been conducted in adults older than 65 years of age. Since renal clearance decreases with age, Cymevene should be administered to geriatric patients with special consideration of their renal status (see Table 1 and section 3.2.5 Pharmacokinetics in Special Populations, Geriatric population – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Renal impairment_ For patients with renal impairment, the dose of Cymevene should be modified as shown in the table below. 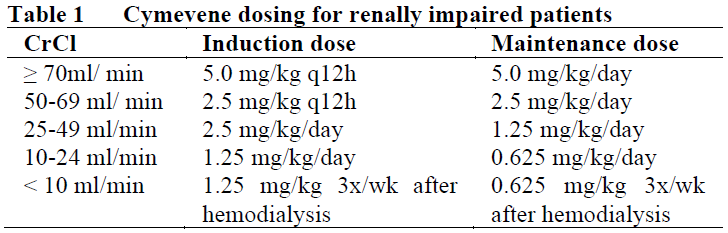 Estimated creatinine clearance can be related to serum creatinine by the following formulae: 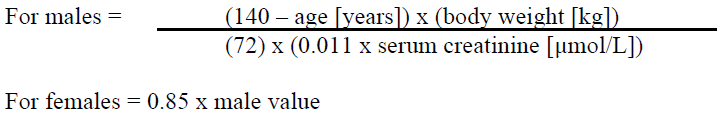 As dosage modifications are recommended in patients with renal impairment, serum creatinine or estimated creatinine-clearance levels should be monitored carefully. _Hepatic impairment_ The safety and efficacy of Cymevene have not been studied in patients with hepatic impairment (see section 3.2.5 Pharmacokinetics in Special Populations, Hepatic impairment – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
INTRAVENOUS
Medical Information
**2.1. THERAPEUTIC INDICATION(S)** Cymevene vials are indicated for the prevention and treatment of life- or sight-threatening cytomegalovirus (CMV) disease in immunocompromised individuals. The benefits of Cymevene for CMV prevention in patients with HIV may be limited.
**2.3. CONTRAINDICATIONS** Cymevene is contraindicated in patients with hypersensitivity to ganciclovir, valganciclovir or to any of the excipients.
J05AB06
ganciclovir
Manufacturer Information
DKSH SINGAPORE PTE. LTD.
BSP Pharmaceuticals S.P.A. (DP + Primary Packager)
Valdepharm (DP + Primary Packager)
Active Ingredients
Documents
Package Inserts
CYMEVENE FOR INJECTION PI.pdf
Approved: December 28, 2021
