Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**4.2. Posology and method of administration** EGFR mutation status should be established prior to initiation of VIZIMPRO therapy. Posology The recommended dose of VIZIMPRO is 45 mg taken orally once daily, until disease progression or unacceptable toxicity occurs. VIZIMPRO can be taken with or without food. Patients should be encouraged to take their dose at approximately the same time each day. If the patient vomits or misses a dose, an additional dose should not be taken and the next prescribed dose should be taken at the usual time the next day. _Dose modifications_ Dose modifications may be required based on individual safety and tolerability. If dose reduction is necessary, then the dose of VIZIMPRO should be reduced as described in Table 1. Dose modification and management guidelines for specific Adverse Drug Reactions (ADRs) are provided in Table 2. No starting dose adjustments are required on the basis of patient age, race, gender, or body weight (see Section 5.2 Pharmacokinetic properties – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). 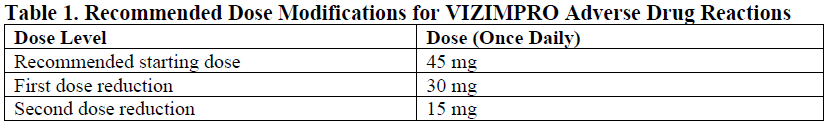 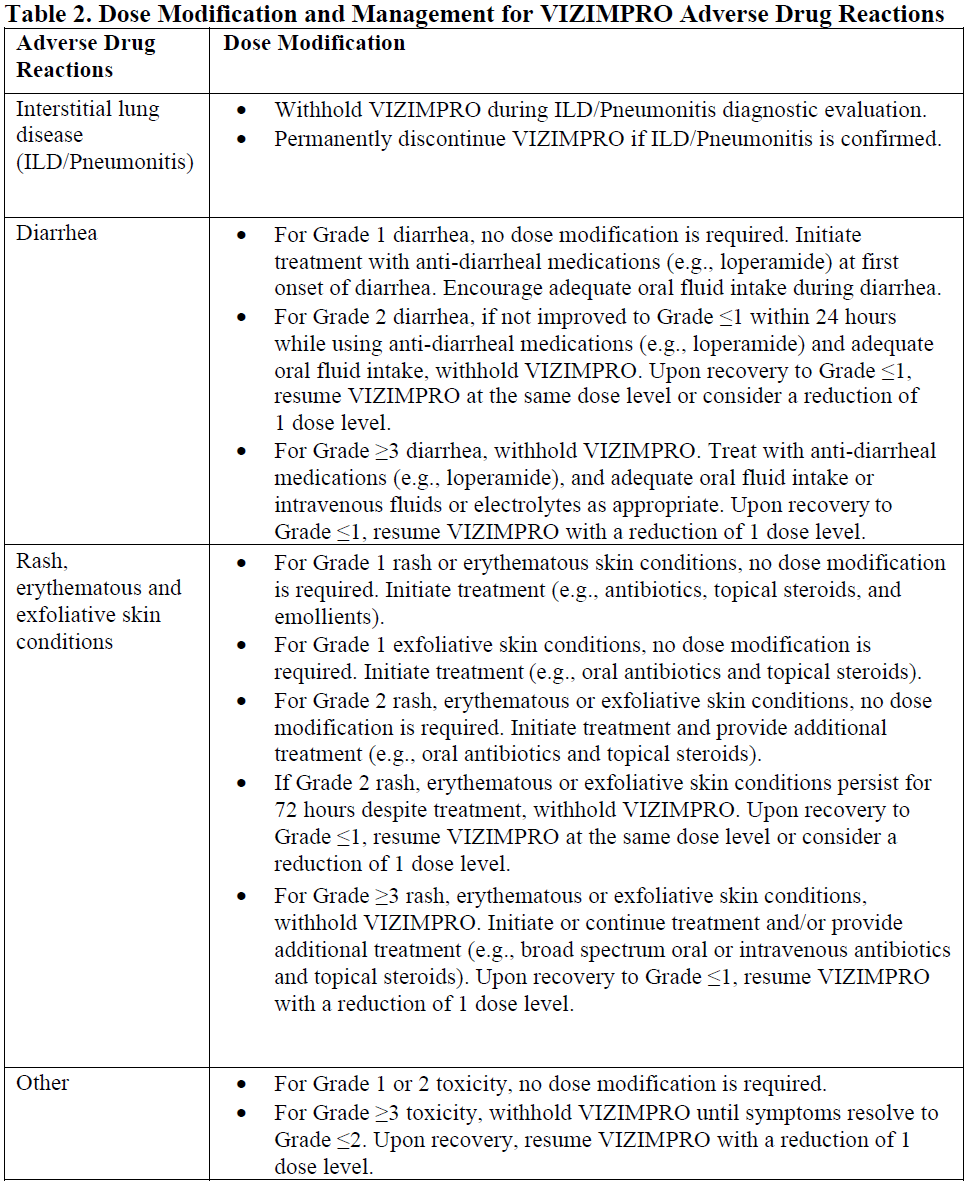 Special populations _Hepatic impairment:_ No starting dose adjustments are required when administering VIZIMPRO to patients with mild (Child-Pugh class A), moderate (Child-Pugh class B) or severe (Child-Pugh class C) hepatic impairment (see Section 5.2 Pharmacokinetic properties – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Renal impairment:_ No starting dose adjustments are required when administering VIZIMPRO to patients with mild or moderate renal impairment (CrCl ≥30 mL/min). Insufficient data are available in patients with severe renal impairment (CrCl <30 mL/min) or requiring hemodialysis to provide dosing recommendations in this patient population (see Section 5.2 Pharmacokinetic properties – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Elderly population:_ No starting dose adjustment of VIZIMPRO in elderly (≥65 years of age) patients is required (see Section 5.2 Pharmacokinetic properties – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Pediatric population:_ The safety and efficacy of VIZIMPRO in children (<18 years of age) have not been established.
ORAL
Medical Information
**4.1. Therapeutic indications** VIZIMPRO is indicated for the first-line treatment of patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR)-activating mutations (exon 19 deletion or exon 21 L858R substitution mutations).
**4.3. Contraindications** Hypersensitivity to the active substance or to any of the excipients.
L01XE47
xl 01 xe 47
Manufacturer Information
PFIZER PRIVATE LIMITED
Pfizer Manufacturing Deutschland GmbH
Active Ingredients
Documents
Package Inserts
Vizimpro film coated tablet PI and PIL.pdf
Approved: January 31, 2022
