Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
CAPSULE
**4.2 Posology and method of administration** LENVIMA treatment should be initiated and supervised by a health care professional experienced in the use of anticancer therapies. Posology _Adults_ Differentiated Thyroid Cancer The recommended daily dose of LENVIMA is 24 mg taken once daily. The daily dose is to be modified as needed according to the dose/toxicity management plan (see dose adjustment section below). Renal Cell Carcinoma _LENVIMA in combination with pembrolizumab_ The recommended daily dose of LENVIMA is 20mg orally once daily in combination with pembrolizumab either 200 mg every 3 weeks or 400 mg every 6 weeks administered as an intravenous infusion over 30 minutes. Refer to the pembrolizumab prescribing information for other pembrolizumab dosing information. _LENVIMA in combination with everolimus_ The recommended daily dose of LENVIMA is 18 mg in combination with 5 mg everolimus orally taken once daily. Refer to the everolimus prescribing information for other everolimus dosing information. Hepatocellular Carcinoma The recommended dosage of LENVIMA is based on actual body weight: - 12 mg for patients greater than or equal to 60 kg or - 8 mg for patients less than 60 kg. Take LENVIMA orally once daily until disease progression or until unacceptable toxicity. Endometrial Carcinoma The recommended dosage of LENVIMA is 20 mg orally once daily, in combination with pembrolizumab either 200 mg every 3 weeks or 400 mg every 6 weeks, administered as an intravenous infusion over 30 minutes, until unacceptable toxicity or disease progression. Refer to the pembrolizumab prescribing information for recommended pembrolizumab dosing information. If a patient misses a dose, and it cannot be taken within 12 hours, then that dose should be skipped and the next dose should be taken at the usual time of administration. Treatment should continue as long as clinical benefit is observed or until unacceptable toxicity occurs. Optimal medical management for nausea, vomiting, and diarrhoea should be initiated prior to any interruption or dose reduction of LENVIMA. Gastrointestinal toxicity should be actively managed in order to reduce the risk of development of renal impairment or failure (see section 4.4 Renal failure and impairment – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Dose adjustment_ Management of adverse reactions may require dose interruption, adjustment, or discontinuation of the combination therapy (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Mild to moderate adverse reactions (e.g., Grade 1 or 2) generally do not warrant interruption of the combination, unless intolerable to the patient despite optimal management. Severe (e.g., Grade 3) or intolerable adverse reactions require interruption of the combination of medicines until improvement of the reaction to Grade 0–1 or baseline. Treatment should be discontinued in case of life-threatening reactions (eg, Grade 4) with the exception of laboratory abnormalities judged to be non-life-threatening, in which case they should be managed as severe reactions (eg, Grade 3). For asymptomatic laboratory abnormalities, such as Grade ≥3 elevations of amylase and lipase that are not considered clinically relevant, lenvatinib continuation without dose modification may be considered. For toxicities thought to be related to lenvatinib (see Table 1), upon resolution/improvement of an adverse reaction to Grade 0–1 or baseline, treatment should be resumed at a reduced dose of lenvatinib as suggested in Table 2. For toxicities thought to be related to everolimus, treatment should be interrupted, reduced to alternate day dosing, or discontinued (see the everolimus SmPC for advice on specific adverse reactions). For toxicities thought to be related to both lenvatinib and everolimus, lenvatinib should be reduced prior to reducing everolimus. When used in combination with pembrolizumab, one or both medicines should be interrupted as appropriate. Lenvatinib should be withheld, dose reduced, or discontinued as appropriate. Withhold or discontinue pembrolizumab in accordance with the instructions in the prescribing information for pembrolizumab. No dose reductions are recommended for pembrolizumab. Grades are based on the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE). Recommendations for LENVIMA dose interruption, reduction and discontinuation for adverse reactions are listed in Table 1. Table 2 lists the recommended dosage reductions of LENVIMA for adverse reactions. 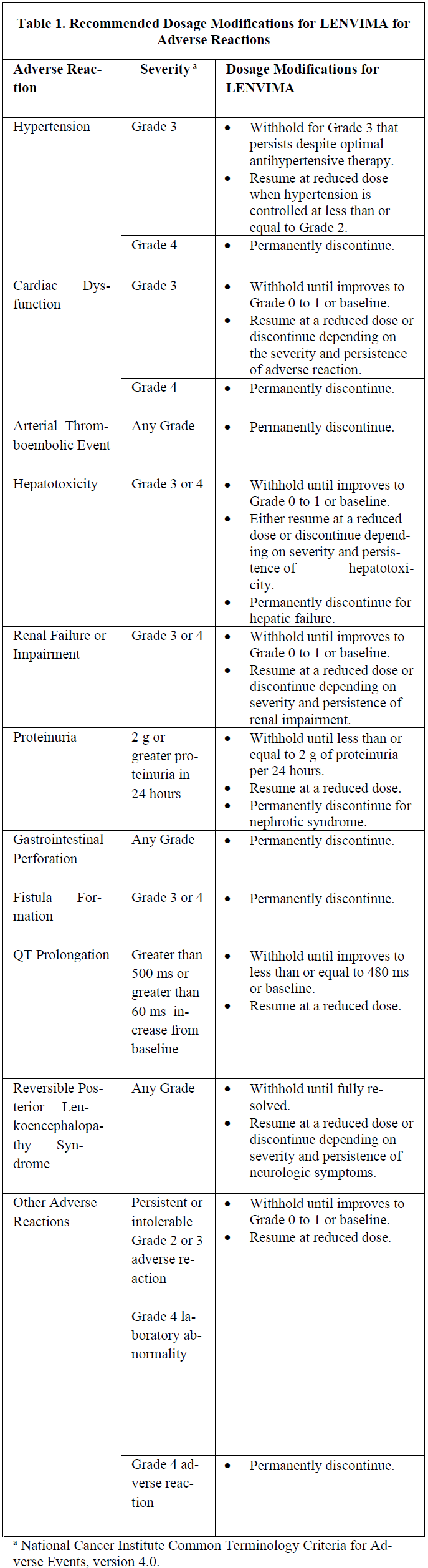 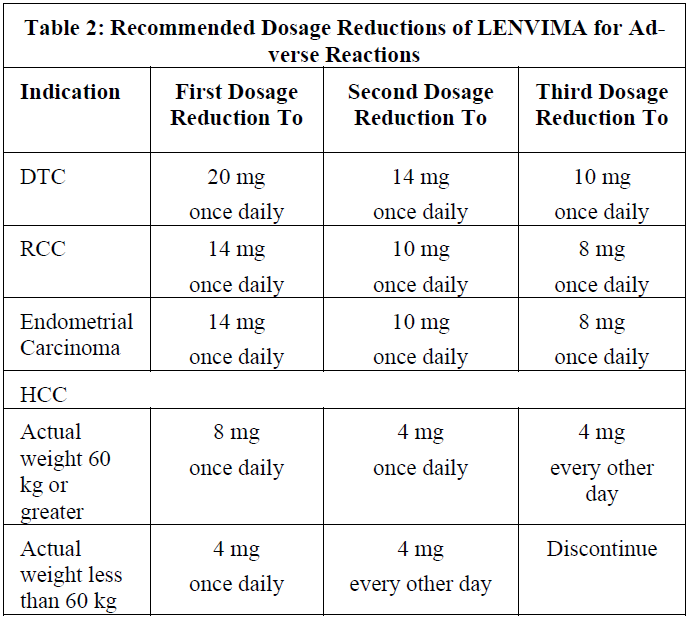 When administering LENVIMA in combination with everolimus for the treatment of renal cell carcinoma, reduce the LENVIMA dose first and then the everolimus dose for adverse reactions of both LENVIMA and everolimus. Refer to the everolimus prescribing information for additional dose modification information. When administering LENVIMA in combination with pembrolizumab for the treatment of endometrial carcinoma, interrupt one or both drugs or dose reduce LENVIMA as appropriate. No dose reductions are recommended for pembrolizumab. Withhold or discontinue pembrolizumab in accordance with the instructions in the pembrolizumab prescribing information. Special populations Patients of age ≥75 years, of Asian race, with comorbidities (such as hypertension, and hepatic or renal impairment), or body weight below 60 kg appear to have reduced tolerability to lenvatinib (see section 4.8e – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). All patients other than those with severe hepatic or renal impairment (see below) should initiate treatment at the recommended 24 mg dose for DTC and 18 mg dose for RCC, following which the dose should be further adjusted on the basis of individual tolerability. _Patients with hypertension_ Blood pressure should be well controlled prior to treatment with lenvatinib, and should be regularly monitored during treatment (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Patients with hepatic impairment_ In patients with DTC, RCC and EC, no adjustment of starting dose is required on the basis of hepatic function in patients with mild (Child-Pugh A) or moderate (Child-Pugh B) hepatic impairment. In patients with severe (Child-Pugh C) hepatic impairment, the recommended starting dose for DTC is 14 mg taken once daily; for RCC and endometrial carcinoma is 10 mg taken once daily. Further dose adjustments may be necessary on the basis of individual tolerability. Limited data are available for the combination of lenvatinib with pembrolizumab or everolimus in patients with hepatic impairment. Please refer to the respective prescribing information for pembrolizumab or everolimus for dosing in patients with hepatic impairment. No dose adjustment is recommended for patients with HCC and mild hepatic impairment (Child-Pugh A). There is no recommended dose for patients with HCC with moderate or severe hepatic impairment. _Patients with renal impairment_ No adjustment of starting dose is required on the basis of renal function in patients with mild or moderate renal impairment. In patients with severe renal impairment, the recommended starting dose for DTC is 14 mg taken once daily; for RCC and endometrial carcinoma is 10 mg taken once daily. Further dose adjustments may be necessary based on individual tolerability. Limited data are available for the combination of lenvatinib with pembrolizumab or everolimus in patients with renal impairment. Please refer to the respective prescribing information for pembrolizumab or everolimus for dosing in patients with renal impairment. There is no recommended dose of LENVIMA for patients with HCC and severe renal impairment. Patients with end-stage renal disease were not studied, therefore the use of lenvatinib in these patients is not recommended. _Elderly population_ No adjustment of starting dose is required on the basis of age. _Paediatric population_ Lenvatinib must not be used in children younger than 2 years of age because of safety concerns regarding organ growth and maturation (see section 5.3 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The safety and efficacy of lenvatinib in children aged 2 to <18 years have not yet been established (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). No data are available. Method of administration Lenvatinib should be taken at about the same time each day, with or without food (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The capsules should be swallowed whole with water. Alternatively, the capsules can be dissolved in a small glass of liquid. Measure 1 tablespoon of water or apple juice and put the capsules into the liquid without breaking or crushing them. Leave the capsules in the liquid for at least 10 minutes. Stir for at least 3 minutes. Drink the mixture. After drinking, add the same amount (1 tablespoon) of water or apple juice to the glass. Swirl the contents a few times and swallow the additional liquid.
ORAL
Medical Information
**4.1 Therapeutic indications** Differentiated Thyroid Cancer (DTC) LENVIMA is indicated for the treatment of patients with locally recurrent or metastatic, progressive, radioactive iodine-refractory differentiated thyroid cancer. Renal Cell Carcinoma (RCC) LENVIMA, in combination with pembrolizumab, is indicated for the first-line treatment of patients with advanced renal cell carcinoma (RCC). LENVIMA is indicated in combination with everolimus for the treatment of patients with advanced renal cell carcinoma (RCC) following one prior vascular endothelial growth factor (VEGF)-targeted therapy. Hepatocellular Carcinoma (HCC) LENVIMA is indicated for the first-line treatment of patients with unresectable hepatocellular carcinoma (HCC). Endometrial Carcinoma (EC) LENVIMA, in combination with pembrolizumab, is indicated for the treatment of adult patients with advanced or recurrent endometrial carcinoma (EC) who have disease progression on or following prior treatment with a platinum-containing therapy in any setting and are not candidates for curative surgery or radiation.
**4.3 Contraindications** Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
L01XE29, NA
xl 01 xe 29, na
Manufacturer Information
EISAI (SINGAPORE) PTE. LTD.
Toronto Region Operations, Patheon Inc.
Eisai Co., Ltd. (Kawashima Plant) (DP & Primary/Secondary Packager)
Active Ingredients
Documents
Package Inserts
LENVIMA PI.pdf
Approved: October 12, 2022
