Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INFUSION, SOLUTION
**Posology and method of administration** Intravenous use The 100 ml vial is restricted to adults, adolescents and children weighing more than 33 kg (approximately 11 years old). The 50 ml vial is restricted to term newborn infants, infants, toddlers and children weighing up to 33 kg. **Posology** Dosing based on patient weight (please see the dosing table here below): 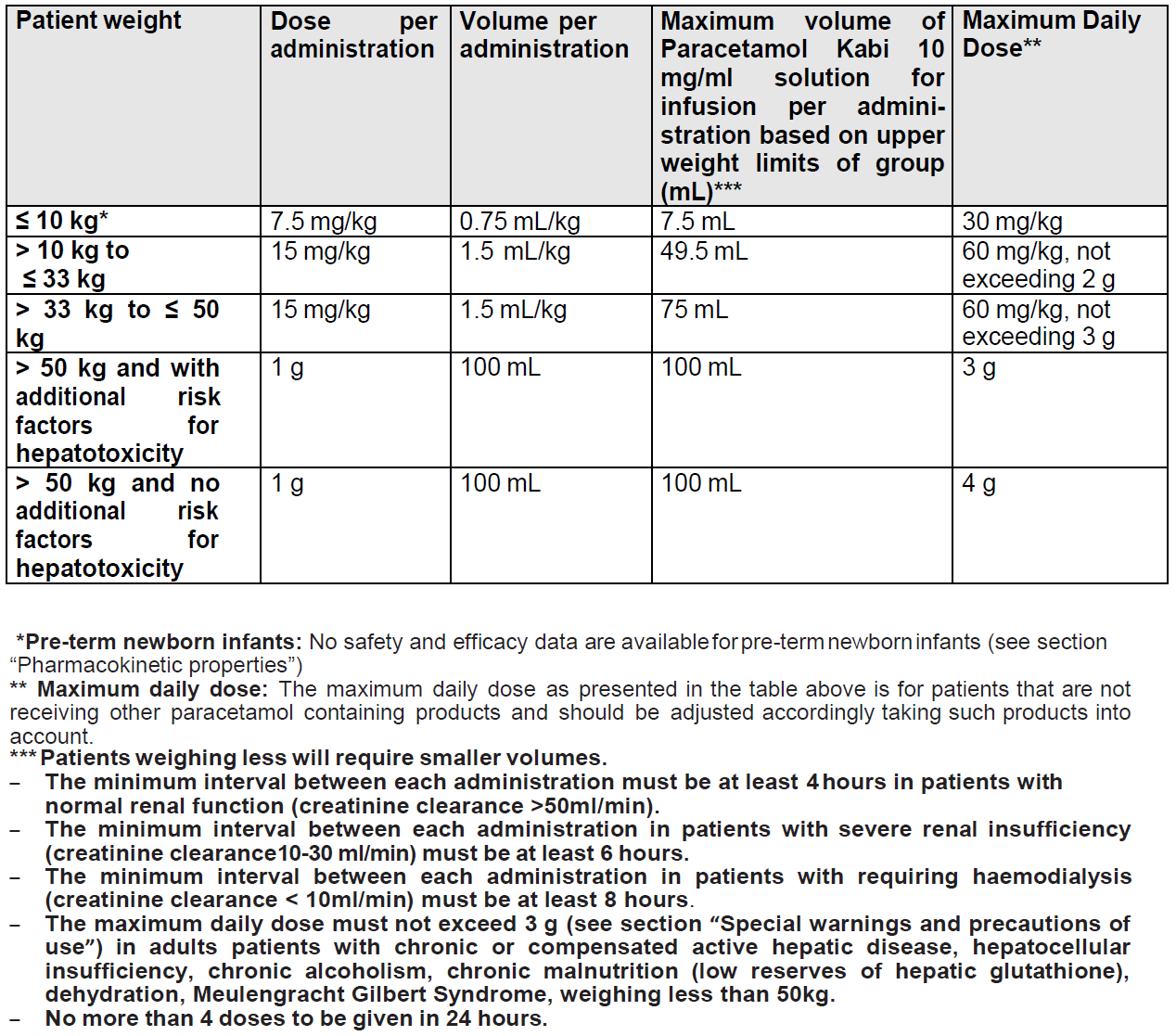 **Method of administration** Take care when prescribing and administering Paracetamol Kabi 10mg/ml solution for infusion to avoid dosing errors due to confusion between milligram (mg) and millilitre (mL), which could result in accidental overdose and death. Take care to ensure the proper dose is communicated and dispensed. When writing prescriptions, include both the total dose in mg and the total dose in volume. Take care to ensure the dose is measured and administered accurately. For single use only. Any unused solution should be discarded. Before administration, the product should be visually inspected for any particulate matter and discolouration. The paracetamol solution is administered as a 15-minute intravenous infusion. Patient weighing ≤ 10 kg: - The glass vial of Paracetamol Kabi 10 mg/ml solution for infusion should not be hung as an infusion due to small volume of medicinal product to be administered in this population. - The volume to be administered should be withdrawn from the vial and diluted in 0.9% sodium chloride solution or 5% glucose solution up to one tenth (one volume Paracetamol Kabi 10 mg/ml solution for infusion into nine volumes diluent) and administered over 15 minutes. Use the diluted solution immediately following its preparation. However, if the diluted solution is not used immediately, do not store for more than 6 hours (infusion time included). - A 5 or 10 mL syringe should be used to measure the dose as appropriate for the weight of the child and the desired volume. However, this should never exceed 7.5 mL per dose - The user should be referred to the product information for dosing guidelines. For dilution of Paracetamol Kabi 10 mg/ml solution for infusion see section ”Pharmaceutical precautions” – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. For the 50ml and 100ml vials: To remove solution, use a 0.8 mm needle (21-gauge needle) and vertically perforate the stopper at the spot specifically indicated. As for all solutions for infusion presented in glass vials, it should be remembered that close monitoring is needed notably at the end of the infusion, regardless of administration route. This monitoring at the end of the perfusion applies particularly for central route infusion, in order to avoid air embolism. For the 50ml vial: Paracetamol Kabi of 50ml vial can also be diluted in a 0.9% sodium chloride solution or 5% glucose solution (from one to nine volumes diluent). In this case, use the diluted solution immediately following its preparation. However, if the diluted solution is not used immediately, do not store for more than 6 hours (infusion time included).
INTRAVENOUS
Medical Information
**Therapeutic indications** Paracetamol Kabi is indicated for: - the short-term treatment of moderate pain, especially following surgery, - the short-term treatment of fever, when administration by intravenous route is clinically justified by an urgent need to treat pain or hyperthermia and/or when other routes of administration are not possible.
**Contraindications** - Hypersensitivity to the active substance, propacetamol hydrochloride (prodrug of paracetamol) or to any of the excipients - Severe hepatocellular insufficiency (Child-Pugh >9)
N02BE01
paracetamol
Manufacturer Information
FRESENIUS KABI (SINGAPORE) PTE LTD
FRESENIUS KABI AUSTRIA GMBH (PLANT GRAZ)
Fresenius Kabi Deutschalnd GmbH
Active Ingredients
Documents
Package Inserts
Paracetamol Kabi Injection PI (Friedberg).pdf
Approved: June 9, 2022
