Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
GEL
**3\. HOW TO USE OESTROGEL® ?** **DOSAGE:** The oestradiol gel is presented in a tube. Each graduated measure delivers 2.5 g of gel, containing 1.5 mg of oestradiol. The average dose is 1 graduated measure per day, during 24 to 28 days per month. Your doctor will prescribe OESTROGEL® according to a scheme adapted to your case. Bleeding resembling menstrual bleeding can occur during the period of discontinuation. This bleeding is normal and is not abundant. If irregular or over-abundant bleeding occurs, consult your doctor. If you have the impression the effect of OESTROGEL® is too strong or too weak, consult your doctor or pharmacist. Estrogen with or without progestogens should be prescribed at the lowest effective doses and for the shortest duration consistent with treatment goals and risks for the individual women. **ADMINISTRATION METHOD AND ROUTE:** Transdermal route. Application of the gel is made over a large surface of clean skin (on the arms, upper part of the bottom, lower part of the abdomen, lumbar region, upper part of the thighs, etc.). The gel should not be applied to the breasts or the mucous membranes. A massage is usefulness but it is recommended to let it dry for about 2 minutes first before putting on clothing. The gel does not leave marks. It is recommended to wash hands after applying the gel. 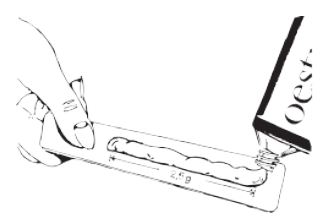 **FREQUENCY AND MOMENT AT WHICH THE MEDICINE SHOULD BE ADMINISTERED:** The application can take place either in the evening or in the morning. **DURATION OF TREATMENT:** According to your doctor’s indications. **STEPS TO TAKE IN THE CASE OF OVERDOSING:** The signs of an overdose are usually a feeling of pain in the breasts, abdominal swelling, flatulence, irritability. No specific treatment is required. These signs disappear when the dose is reduced. If these symptoms persist, consult your doctor. **STEPS TO TAKE IN THE CASE WHERE ONE OR MORE DOSES HAVE BEEN OMITTED:** **If you forget to use OESTROGEL** ®: if you have forgotten to apply the gel on the planned day, do it as quickly as possible and return to the therapeutic schedule as initially intended. _Do not take a double dose to compensate for the single dose you have forgotten to take._ If you have not been treated for several days in a row, irregular bleeding may occur. If you have any doubts, consult your doctor. **RISK OF WITHDRAWAL SYMPTOMS** When the treatment is ended, signs of a deficit in oestrogen linked to the menopause could reappear.
TOPICAL
Medical Information
**1\. WHAT IS OESTROGEL® AND WHAT IT IS USED FOR ?** Gel for cutaneous application, tube of 80 g. ATC classification: G03CA03 OESTROGENS - (G: genital-urinary system and sex hormones). This medicine contains natural oestrogen. It is prescribed: - for the treatment of problems due to a deficiency in oestrogens linked to menopause. It is hormone replacement therapy or HRT. - for the prevention of post-menopausal osteoporosis for women with an increased risk of fracture and showing either an intolerance or a contraindication to other treatments employed in the prevention of osteoporosis. The choice of this treatment should be discussed with your doctor. Experience of this treatment in women above 65 years of age is limited.
**2\. WARNING!** **WHEN SHOULD OESTROGEL® NOT BE USED?** OESTROGEL® SHOULD NO BE USED in the following cases: - history or a current venous or arterial thrombo-embolic diseases (phlebitis, pulmonary embolia, angina pectoris, myocardial infarction, cerebral vascular incidents); - breast cancer, uterine or any other oestrogen-dependent cancer; - endometrial hyperplasia (excessive development of the uterine mucous membrane); - undiagnosed vaginal bleeding; - certain liver illnesses; - known allergies to one of its constituents; - porphyria ( _hereditary disease_).
G03CA03
estradiol
Manufacturer Information
PHARMED IMPORT & EXPORT PTE LTD
Besins Manufacturing Belgium
Laboratoires Besins International
Active Ingredients
Documents
Package Inserts
Oestrogel PI.pdf
Approved: February 16, 2022
