Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION
**IV. DOSAGE AND ADMINISTRATION** **General Recommendations in Adult Patients** CASPOCAN should be administered in adults (≥ 18 years of age) by slow intravenous infusion over approximately 1 hour. Empirical Therapy A single 70-mg loading dose should be administered on Day 1, followed by 50 mg daily thereafter. Duration of treatment should be based on the patient’s clinical response. Empirical therapy should be continued until up to 72 hours after resolution of neutropenia. Patients found to have a fungal infection should be treated for a minimum of 14 days; treatment should continue for at least 7 days after both neutropenia and clinical symptoms are resolved. If the 50-mg dose is well tolerated but does not provide an adequate clinical response, the daily dose can be increased to 70 mg. Although an increase in efficacy with 70 mg daily has not been demonstrated, safety data suggest that an increase in dose to 70 mg daily is well tolerated. Candidemia and other _Candida_ infections A single 70-mg loading dose should be administered on Day 1, followed by 50 mg daily thereafter. Duration of treatment should be dictated by the patient’s clinical and microbiological response. In general, antifungal therapy should continue for at least 14 days after the last positive culture. Patients who remain persistently neutropenic may warrant a longer course of therapy pending resolution of the neutropenia. Esophageal Candidiasis Fifty (50) mg should be administered daily. Invasive Aspergillosis A single 70-mg loading dose should be administered on Day 1, followed by 50 mg daily thereafter. Duration of treatment should be based upon the severity of the patient’s underlying disease, recovery from immunosuppression, and clinical response. The efficacy of a 70-mg dose regimen in patients who are not clinically responding to the 50-mg daily dose is not known. Safety data suggest that an increase in dose to 70 mg daily is well tolerated. The efficacy of doses above 70 mg has not been adequately studied in patients with invasive aspergillosis. No dosage adjustment is necessary for elderly patients (65 years of age or more). No dosage adjustment is necessary based on gender, race, or renal impairment. When co-administering CASPOCAN in adult patients with the metabolic inducers efavirenz, nevirapine, rifampicin, dexamethasone, phenytoin, or carbamazepine, use of a daily dose of 70 mg CASPOCAN should be considered. **Patients with Hepatic Insufficiency** Adult patients with mild hepatic insufficiency (Child-Pugh score 5 to 6) do not need a dosage adjustment. For adult patients with moderate hepatic insufficiency (Child-Pugh score 7 to 9), CASPOCAN 35 mg daily is recommended based upon pharmacokinetic data. However, where recommended, a 70-mg loading dose should still be administered on Day 1. There is no clinical experience in adult patients with severe hepatic insufficiency (Child-Pugh score greater than 9) and in pediatric patients with any degree of hepatic insufficiency. **Pediatric Patients** CASPOCAN should be administered in pediatric patients (12 months to 17 years of age) by slow IV infusion over approximately 1 hour. Dosing in pediatric patients (12 months to 17 years of age) should be based on the patient’s body surface area (see Instructions for Use in Pediatric Patients, Mosteller 1 Formula). For all indications with the exception of esophageal candidiasis, a single 70-mg/m2 loading dose (not to exceed an actual dose of 70 mg) should be administered on Day 1, followed by 50 mg/m2 daily thereafter (not to exceed an actual dose of 70 mg daily). For the treatment of esophageal candidiasis, a dose of 50mg/m2 daily should be administered (not to exceed an actual dose of 70mg daily). Duration of treatment should be individualized to the indication, as described for each indication in adults (see General Recommendations in Adult Patients). If the 50-mg/m2 daily dose is well tolerated but does not provide an adequate clinical response, the daily dose can be increased to 70 mg/m2 daily (not to exceed an actual daily dose of 70 mg). Although an increase in efficacy with 70 mg/m2 daily has not been demonstrated, limited safety data suggest that an increase in dose to 70 mg/m2 daily is well tolerated. When CASPOCAN is co-administered to pediatric patients with inducers of drug clearance, such as rifampin, efavirenz, nevirapine, phenytoin, dexamethasone, or carbamazepine, use of a CASPOCAN dose of 70-mg/m2 daily (not to exceed an actual daily dose of 70 mg) should be considered. **Reconstitution of CASPOCAN** DO NOT USE ANY DILUENTS CONTAINING DEXTROSE (α-D-GLUCOSE), as CASPOCAN is not stable in diluents containing dextrose. DO NOT MIX OR CO-INFUSE CASPOCAN WITH ANY OTHER MEDICATIONS, as there are no data available on the compatibility of CASPOCAN with other intravenous substances, additives, or medications. Visually inspect the infusion solution for particulate matter or discoloration. **INSTRUCTIONS FOR USE IN ADULTS** **Step 1 Reconstitution of conventional vials** To reconstitute the powdered drug, bring the refrigerated conventional vial of CASPOCAN to room temperature and aseptically add 10.5 mL of either 0.9% Sodium Chloride Injection, Sterile Water for Injection, Bacteriostatic Water for Injection with methylparaben and propylparaben, or Bacteriostatic Water for Injection with 0.9% benzyl alcohol. The concentrations of the reconstituted vials will be: 7.2 mg/mL (70 mg vial) or 5.2 mg/mL (50 mg vial). The white to off-white compact powder will dissolve completely. Mix gently until a clear solution is obtained. Reconstituted solutions should be visually inspected for particulate matter or discoloration. This reconstituted solution may be stored for up to 24 hours at or below 25°C (77°F). **Step 2 Addition of Reconstituted CASPOCAN to patient infusion solution** Diluents for the final patient infusion solutions are: Sterile Saline for Injection, or Lactated Ringer’s Solution. The standard patient infusion is prepared by aseptically adding the appropriate amount of reconstituted drug (as shown in the table below) to a 250 mL intravenous bag or bottle. Reduced volume infusions in 100 mL may be used, when medically necessary, for 50 mg or 35 mg daily doses. Do not use if the solution is cloudy or precipitated. This infusion solution must be used within 24 hours if stored at or below 25°C (77°F) or within 48 hours if stored refrigerated at 2 to 8°C (36 to 46°F). CASPOCAN should be administered by slow intravenous infusion over approximately 1 hour. 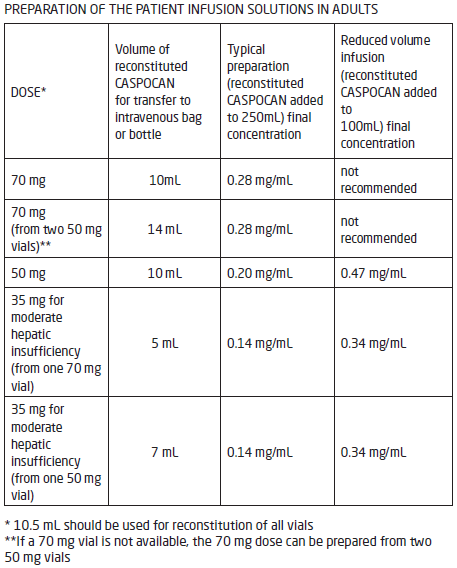 **INSTRUCTIONS FOR USE IN PEDIATRIC PATIENTS** Calculation of Body Surface Area (BSA) for pediatric dosing Before preparation of infusion, calculate the body surface area (BSA) of the patient using the following formula: (Mosteller Formula): 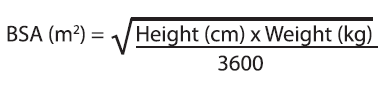 Preparation of the 70-mg/m2 infusion for pediatric patients 12 months of age or older (using a 70-mg vial) 1. Determine the actual loading dose to be used in the pediatric patient by using the patient’s BSA (as calculated above) and the following equation: 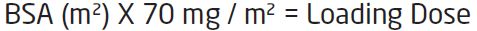 The maximum loading dose on Day 1 should not exceed 70 mg regardless of the patient’s calculated dose. 2. Equilibrate the refrigerated vial of CASPOCAN to room temperature. 3. Aseptically add 10.5 mL of 0.9% Sodium Chloride Injection, Sterile Water for Injection, Bacteriostatic Water for Injection with methylparaben and propylparaben, or Bacteriostatic Water for Injection with 0.9% benzyl alcohol.a This reconstituted solution may be stored for up to 24 hours at or below 25°C (≤ 77°F).b This will give a final caspofungin concentration in the vial of 7.2 mg/mL. 4. Remove the volume of drug equal to the calculated loading dose (Step 1) from the vial. Aseptically transfer this volume (mL)c of reconstituted CASPOCAN to an IV bag (or bottle) containing 250 mL of 0.9%, 0.45%, or 0.225% Sodium Chloride Injection, or Lactated Ringers Injection. Alternatively, the volume (mL)c of reconstituted CASPOCAN can be added to a reduced volume of 0.9%, 0.45%, or 0.225% Sodium Chloride Injection or Lactated Ringers Injection, not to exceed a final concentration of 0.5 mg/mL. This infusion solution must be used within 24 hours if stored at or below 25°C (≤ 77°F) or within 48 hours if stored refrigerated at 2 to 8°C (36 to 46°F). 5. If the calculated loading dose is <50 mg, then the dose may be prepared from the 50-mg vial \[follow Steps 2–4 from Preparation of the 50-mg/m2 infusion for pediatric patients 12 months of age or older (using a 50-mg vial)\]. The final caspofungin concentration in the 50-mg vial after reconstitution is 5.2 mg/mL. Preparation of the 50 mg/m2 infusion for pediatric patients 12 months of age or older (using a 50 mg vial) 1. Determine the daily maintenance dose to be used in the pediatric patient by using the patient’s BSA (as calculated above) and the following equation: 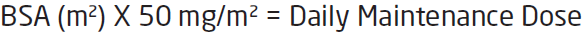 The daily maintenance dose should not exceed 70 mg regardless of the patient’s calculated dose. 2. Equilibrate the refrigerated vial of CASPOCAN to room temperature. 3. Aseptically add 10.5 mL of 0.9% Sodium Chloride Injection, Sterile Water for Injection, Bacteriostatic Water for Injection with methylparaben and propylparaben, or Bacteriostatic Water for Injection with 0.9% benzyl alcohol.a This reconstituted solution may be stored for up to 24 hours at or below 25°C (≤ 77°F).b This will give a final caspofungin concentration in the vial of 5.2 mg/mL. 4. Remove the volume of drug equal to the calculated loading dose (Step 1) from the vial. Aseptically transfer this volume (mL)c of reconstituted CASPOCAN to an IV bag (or bottle) containing 250 mL of 0.9%, 0.45%, or 0.225% Sodium Chloride Injection, or Lactated Ringers Injection. Alternatively, the volume (mL)c of reconstituted CASPOCAN can be added to a reduced volume of 0.9%, 0.45%, or 0.225% Sodium Chloride Injection or Lactated Ringers Injection, not to exceed a final concentration of 0.5 mg/mL. This infusion solution must be used within 24 hours if stored at or below 25°C (≤ 77°F) or within 48 hours if stored refrigerated at 2 to 8°C (36 to 46°F). 5. If the actual daily maintenance dose is >50 mg, then the dose may be prepared from the 70-mg vial \[follow Steps 2–4 from Preparation of the 70-mg/m2 infusion for pediatric patients 12 months of age or older (using a 70-mg vial)\]. The final caspofungin concentration in the 70-mg vial after reconstitution is 7.2 mg/mL. Preparation notes: a. The white to off-white cake will dissolve completely. Mix gently until a clear solution is obtained. b. Visually inspect the reconstituted solution for particulate matter or discoloration during reconstitution and prior to infusion. Do not use if the solution is cloudy or has precipitated. c. CASPOCAN is formulated to provide the full labeled vial dose (70 mg or 50 mg) when 10mL is withdrawn from the vial.
INTRAVENOUS
Medical Information
**III. INDICATIONS** CASPOCAN is indicated in adult and pediatric patients (12 months and older) for: - Empirical therapy for presumed fungal infections (such as _Candida_ or _Aspergillus_) in febrile, neutropenic patients, whose fever has failed to respond to broad-spectrum antibiotics. - Treatment of Candidemia and the following _Candida_ infections: intraabdominal abscesses, peritonitis and pleural space infections. CASPOCAN has not been studied in endocarditis, osteomyelitis, and meningitis due to _Candida_. - Treatment of Esophageal Candidiasis. - Treatment of Invasive Aspergillosis in patients who are refractory to or intolerant of other therapies.
**V. CONTRAINDICATIONS** CASPOCAN is contraindicated in patients with hypersensitivity to any component of this product.
J02AX04
caspofungin
Manufacturer Information
TEVA PHARMACEUTICAL INVESTMENTS SINGAPORE PTE. LTD.
MEFAR İLAÇ SANAYİİ A.Ş.
ELPEN PHARMACEUTICAL CO.,INC
Active Ingredients
Documents
Package Inserts
Caspofungin_PI_ELPEN_Approved.pdf
Approved: June 27, 2022
