Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, POWDER, FOR SOLUTION
**4.2 Posology and method of administration** **Posology** _Adults and the elderly population_ The following administered doses are recommended (other doses may be justifiable). _Intravenous use_ - Measuring glomerular filtration rate from plasma: 7 – 18 MBq. - Renal scintigraphy: 40 – 400 MBq. - Cerebral angioscintigraphy: 185 – 740 MBq. _For inhalation_ Lung ventilation imaging: - 500 – 1000 MBq deposited in a nebuliser - 50 – 100 MBq in the lung. _For oral use_ - Detection of gastroesophageal reflux and gastric emptying study: 10 – 20 MBq. - Technetium (99mTc) pentetate is mixed with an appropriate volume (30 to 240 mL) of liquid carrier (e.g. milk). _Renal/ hepatic impairment_ Careful consideration of the activity to be administered is required since an increased radiation exposure is possible in these patients (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Paediatric population_ The use in children and adolescents has to be considered carefully, based upon clinical needs and assessing the risk/benefit ratio in this patient group. The activities to be administered by intravenous route to children and to adolescents may be calculated according to the recommendations of the European Association of Nuclear Medicine EANM (2016) paediatric dosage card, by using the formula corresponding to the concerned indication and the relevant correction factor corresponding to the body mass of the young patient. - Administration of technetium (99mTc) pentetate in abnormal renal function: Administered Activity\[MBq\] = Baseline Activity x Multiple (with a baseline activity of 14.0) 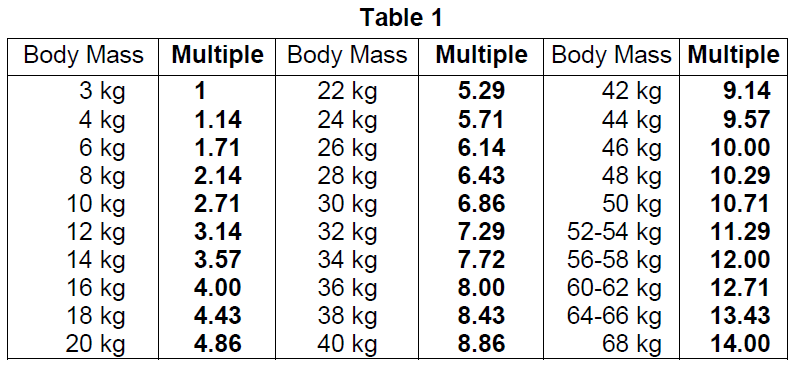 In very young children (up to 1 year), when technetium (99mTc) pentetate is used for urinary tract examinations, a minimum dose of 20 MBq is necessary to obtain images of sufficient quality. - Administration of technetium (99mTc) pentetate in normal renal function: Administered Activity\[MBq\] = Baseline Activity x Multiple (with a baseline activity of 34.0) 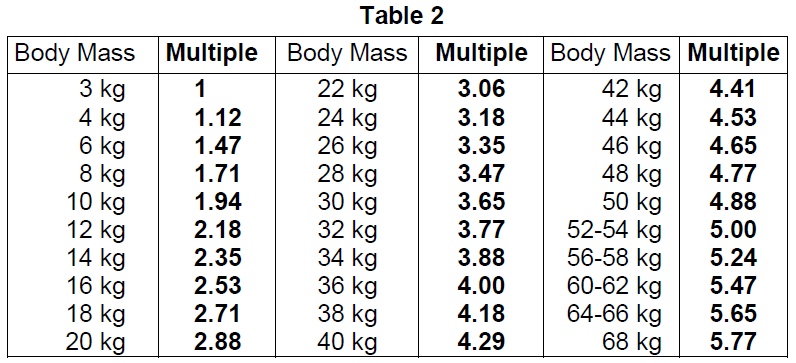 - Lung ventilation imaging: 500 – 1000 MBq deposited in the nebuliser; 10 MBq in the lung. - Detection of gastroesophageal reflux and liquid gastric emptying study: 10 – 20 MBq. Administered activity of the radiopharmaceutical and the volume to be fed to the patient should be based on patient factors such as age, body weight, and the usual feeding volume. Administered activity for children should be as low as reasonably achievable for diagnostic image quality. **Method of administration** For intravenous, inhalation and oral administration. For multidose use. This medicinal product should be reconstituted before administration to the patient. For instructions on reconstitution and radiolabelling of the medicinal product before administration, see section 12 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. For patient preparation, see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. **Image acquisition** - Renal perfusion imaging is obtained by dynamic acquisitions immediately after injection up to 1 minute. The optimal static imaging time is 1 hour post injection. In case of captopril (ACE inhibitor) renography, captopril is given intravenously before technetium (99mTc) pentetate administration. Individual kidney function and urinary outflow imaging are obtained by dynamic acquisitions performed after injection. If one or both kidneys have not emptied satisfactorily during the first 20 minutes, a furosemide challenge is performed and the dynamic acquisition should continue for a further 15-minute after the diuretic. Static images may be acquired 1 hour after injection. - For cerebral examinations, dynamic acquisitions should begin immediately after injection. Static images are obtained 1 hour and, if necessary, several hours after injection. - For lung ventilation imaging: images of the lungs are obtained during 180 min. Dynamic images of oesophagus are obtained during the first minutes after administration followed by continuous imaging for 60 minutes for evaluation of gastroesophageal reflux. Gastric emptying at 60 minutes and at 2 or 3 hours after completion of feeding is calculated.
INTRAVENOUS
Medical Information
**4.1 Therapeutic indications** This medicinal product is for diagnostic use only. After reconstitution with sodium pertechnetate (99mTc) solution, the solution of technetium (99mTc) pentetate is indicated for: 1. After intravenous administration for: - Measurement of glomerular filtration rate. - Renal perfusion and function and urinary tract studies. - Cerebral angioscintigraphy (as an alternative method when computed tomography and/or magnetic resonance imaging are not available). 2. After inhalation of the nebulized technetium (99mTc) pentetate for: - Lung ventilation imaging. 3. After oral administration of the technetium (99mTc) pentetate for: - Detection of gastroesophageal reflux and liquid gastric emptying study.
**4.3 Contraindications** Hypersensitivity to the active substance, or to any of the excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
NA
xna
Manufacturer Information
QT INSTRUMENTS (S) PTE LTD
Curium Netherlands B.V.
Active Ingredients
Documents
Package Inserts
05 CON 4362 Singapore PI 15052019_clean.doc
Approved: June 29, 2020
