Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INFUSION, SOLUTION
**4.2 Posology and method of administration** Filgrastim therapy should only be given in collaboration with an oncology centre which has experience in G-CSF treatment and haematology and has the necessary diagnostic facilities. The mobilisation and apheresis procedures should be performed in collaboration with an oncology-haematology centre with acceptable experience in this field and where the monitoring of haematopoietic progenitor cells can be correctly performed. Established cytotoxic chemotherapy _Posology_ The recommended dose of filgrastim is 0.5 million units (5 mcg)/kg/day. The first dose of filgrastim should be administered at least 24 hours after cytotoxic chemotherapy. In randomised clinical trials, a subcutaneous dose of 230 mcg/m2/day (4.0 to 8.4 mcg/kg/day) was used. Daily dosing with filgrastim should continue until the expected neutrophil nadir is passed and the neutrophil count has recovered to the normal range. Following established chemotherapy for solid tumours, lymphomas, and lymphoid leukaemia, it is expected that the duration of treatment required to fulfil these criteria will be up to 14 days. Following induction and consolidation treatment for acute myeloid leukaemia the duration of treatment may be substantially longer (up to 38 days) depending on the type, dose and schedule of cytotoxic chemotherapy used. In patients receiving cytotoxic chemotherapy, a transient increase in neutrophil counts is typically seen 1 to 2 days after initiation of filgrastim therapy. However, for a sustained therapeutic response, filgrastim therapy should not be discontinued before the expected nadir has passed and the neutrophil count has recovered to the normal range. Premature discontinuation of filgrastim therapy, prior to the time of the expected neutrophil nadir, is not recommended. _Method of administration_ Filgrastim may be given as a daily subcutaneous injection or as a daily intravenous infusion diluted in 5% glucose solution given over 30 minutes (see section 6.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The subcutaneous route is preferred in most cases. There is some evidence from a study of single dose administration that intravenous dosing may shorten the duration of effect. The clinical relevance of this finding to multiple dose administration is not clear. The choice of route should depend on the individual clinical circumstance. In patients treated with myeloablative therapy followed by bone marrow transplantation _Posology_ The recommended starting dose of filgrastim is 1.0 million units (10 mcg)/kg/day. The first dose of filgrastim should be administered at least 24 hours following cytotoxic chemotherapy and at least 24 hours after bone marrow infusion. Once the neutrophil nadir has been passed, the daily dose of filgrastim should be titrated against the neutrophil response as follows: 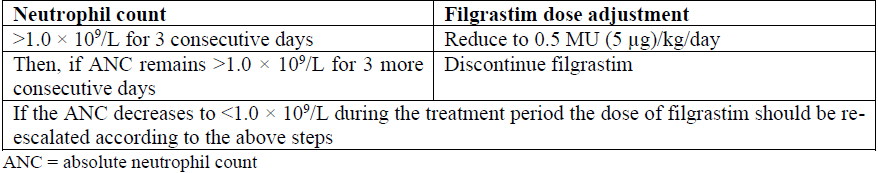 _Method of administration_ Filgrastim may be given as a 30 minute or 24 hour intravenous infusion or given by continuous 24 hour subcutaneous infusion. Filgrastim should be diluted in 20 mL of 5% glucose solution (see section 6.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). For the mobilisation of PBPCs in patients undergoing myelosuppressive or myeloablative therapy followed by autologous PBPC transplantation _Posology_ The recommended dose of filgrastim for PBPC mobilisation when used alone is 1.0 million units (10 mcg)/kg/day for 5 to 7 consecutive days. Timing of leukapheresis: one or two leukapheresis on days 5 and 6 are often sufficient. In other circumstances, additional leukapheresis may be necessary. Filgrastim dosing should be maintained until the last leukapheresis. The recommended dose of filgrastim for PBPC mobilisation after myelosuppressive chemotherapy is 0.5 million units (5 mcg)/kg/day from the first day after completion of chemotherapy until the expected neutrophil nadir is passed and the neutrophil count has recovered to the normal range. Leukapheresis should be performed during the period when the ANC rises from <0.5 × 109/L to >5.0 × 109/L. For patients who have not had extensive chemotherapy, one leukapheresis is often sufficient. In other circumstances, additional leukapheresis is recommended. _Method of administration_ Filgrastim for PBPC mobilisation when used alone: Filgrastim may be given as a 24 hour subcutaneous continuous infusion or subcutaneous injection. For infusions, filgrastim should be diluted in 20 mL of 5% glucose solution (see section 6.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Filgrastim for PBPC mobilisation after myelosuppressive chemotherapy: Filgrastim should be given by subcutaneous injection. For the mobilisation of PBPCs in normal donors prior to allogeneic PBPC transplantation _Posology_ For PBPC mobilisation in normal donors, filgrastim should be administered at 1.0 million units (10 mcg)/kg/day for 4 to 5 consecutive days. Leukapheresis should be started at Day 5 and continued until Day 6 if needed in order to collect 4 × 106 CD34+ cells/kg recipient bodyweight. _Method of administration_ Filgrastim should be given by subcutaneous injection. In patients with severe chronic neutropenia (SCN) _Posology_ Congenital neutropenia: The recommended starting dose is 1.2 million units (12 mcg)/kg/day as a single dose or in divided doses. Idiopathic or cyclic neutropenia: The recommended starting dose is 0.5 million units (5 mcg)/kg/day as a single dose or in divided doses. Dose adjustment: Filgrastim should be administered daily by subcutaneous injection until the neutrophil count has reached and can be maintained at more than 1.5 × 109/L. When the response has been obtained the minimal effective dose to maintain this level should be established. Long term daily administration is required to maintain an adequate neutrophil count. After one to two weeks of therapy, the initial dose may be doubled or halved depending upon the patient’s response. Subsequently the dose may be individually adjusted every 1 to 2 weeks to maintain the average neutrophil count between 1.5 × 109/L and 10 × 109/L. A faster schedule of dose escalation may be considered in patients presenting with severe infections. In clinical trials, 97% of patients who responded had a complete response at doses ≤24 mcg/kg/day. The long-term safety of filgrastim administration above 24 mcg/kg/day in patients with SCN has not been established. _Method of administration_ Congenital, idiopathic or cyclic neutropenia: Filgrastim should be given by subcutaneous injection. In patients with HIV infection _Posology_ For reversal of neutropenia: The recommended starting dose of filgrastim is 0.1 million units (1 mcg)/kg/day with titration up to a maximum of 0.4 million units (4 mcg)/kg/day until a normal neutrophil count is reached and can be maintained (ANC >2.0 × 109/L). In clinical studies, >90% of patients responded at these doses, achieving reversal of neutropenia in a median of 2 days. In a small number of patients (<10%), doses up to 1.0 million units (10 mcg)/kg/day were required to achieve reversal of neutropenia. For maintaining normal neutrophil counts: When reversal of neutropenia has been achieved, the minimal effective dose to maintain a normal neutrophil count should be established. Initial dose adjustment to alternate day dosing with 30 million units (300 mcg)/day by subcutaneous injection is recommended. Further dose adjustment may be necessary, as determined by the patient’s ANC, to maintain the neutrophil count at >2.0 × 109/L. In clinical studies, dosing with 30 million units (300 mcg)/day on 1 to 7 days per week was required to maintain the ANC >2.0 × 109/L, with the median dose frequency being 3 days per week. Long term administration may be required to maintain the ANC >2.0 × 109/L. _Method of administration_ Reversal of neutropenia or maintaining normal neutrophil counts: filgrastim should be given by subcutaneous injection. Elderly Clinical trials with filgrastim have included a small number of elderly patients but special studies have not been performed in this group and therefore specific dosage recommendations cannot be made. Renal or hepatic impairment Studies of filgrastim in patients with severe impairment of renal or hepatic function demonstrate that it exhibits a similar pharmacokinetic and pharmacodynamic profile to that seen in normal individuals. Dose adjustment is not required in these circumstances. Paediatric use in the SCN and cancer settings Sixty-five percent of the patients studied in the SCN trial program were under 18 years of age. The efficacy of treatment was clear for this age group, which included most patients with congenital neutropenia. There were no differences in the safety profiles for paediatric patients treated for SCN. Data from clinical studies in paediatric patients indicate that the safety and efficacy of filgrastim are similar in both adults and children receiving cytotoxic chemotherapy. The dosage recommendations in paediatric patients are the same as those in adults receiving myelosuppressive cytotoxic chemotherapy.
SUBCUTANEOUS, INTRAVENOUS DRIP
Medical Information
**4.1 Therapeutic indications** Filgrastim is indicated for the reduction in the duration of neutropenia and the incidence of febrile neutropenia in patients treated with established cytotoxic chemotherapy for malignancy (with the exception of chronic myeloid leukaemia and myelodysplastic syndromes) and for the reduction in the duration of neutropenia in patients undergoing myeloablative therapy followed by bone marrow transplantation considered to be at increased risk of prolonged severe neutropenia. The safety and efficacy of filgrastim are similar in adults and children receiving cytotoxic chemotherapy. Filgrastim is indicated for the mobilisation of peripheral blood progenitor cells (PBPCs). In patients, children or adults, with severe congenital, cyclic, or idiopathic neutropenia with an absolute neutrophil count (ANC) of ≤0.5 × 109/L, and a history of severe or recurrent infections, long term administration of filgrastim is indicated to increase neutrophil counts and to reduce the incidence and duration of infection-related events. Filgrastim is indicated for the treatment of persistent neutropenia (ANC less than or equal to 1.0 × 109/L) in patients with advanced HIV infection, in order to reduce the risk of bacterial infections when other options to manage neutropenia are inappropriate.
**4.3 Contraindications** Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
L03AA02
filgrastim
Manufacturer Information
PFIZER PRIVATE LIMITED
Hospira Zagreb d.o.o. (PBF site)
Hospira Zagreb d.o.o. (SM site)
Active Ingredients
Documents
Package Inserts
Nivestim 300mcg 0.5mL PI.pdf
Approved: April 17, 2023
