Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**Dosage and Administration** Pharmaceutical form: Film-coated tablets. **Posology** _TIVICAY_ therapy should be initiated by a physician experienced in the management of HIV infection. _TIVICAY_ can be taken with or without food. **Method of Administration** **Adults** **Patients infected with HIV-1 without documented or clinically suspected resistance to the integrase class** The recommended dose of _TIVICAY_ is 50 mg once daily. _TIVICAY_ should be administered twice daily in this population when co-administered with some medicines (e.g. efavirenz, nevirapine, tipranavir/ritonavir, or rifampicin) (see _Interactions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Patients infected with HIV-1 with resistance to the integrase class (documented or clinically suspected)** The recommended dose of _TIVICAY_ is 50 mg twice daily. The decision to use _TIVICAY_ for such patients should be informed by the integrase resistance pattern (see _Clinical Studies_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Co-administration of _TIVICAY_ with some medicines should be avoided in this population (e.g. efavirenz, nevirapine, tipranavir/ritonavir, or rifampicin) (see _Warnings and Precautions_ and _Interactions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Adolescents** In patients who have not previously been treated with an integrase inhibitor, (12 to less than 18 years of age and weighing greater than or equal to 40 kg) the recommended dose of _TIVICAY_ is 50 mg once daily. _TIVICAY_ should be administered twice daily in this population when co-administered with some medicines (e.g. efavirenz, nevirapine, tipranavir/ritonavir, or rifampicin) (see _Interactions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). There are insufficient data to recommend a dose for _TIVICAY_ in integrase inhibitor resistant adolescents under 18 years of age. **Children** In patients infected with HIV-1 who have not previously been treated with an integrase inhibitor, the recommended dose of _TIVICAY_ in children (6 to less than 12 years of age) is determined according to the weight of the child. Dose recommendations according to weight are presented in the table below. The weight-based once daily dose of _TIVICAY_ should be administered twice daily in this population when co-administered with some medicines (e.g. efavirenz, nevirapine, tipranavir/ritonavir, or rifampicin) (see _Interactions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). 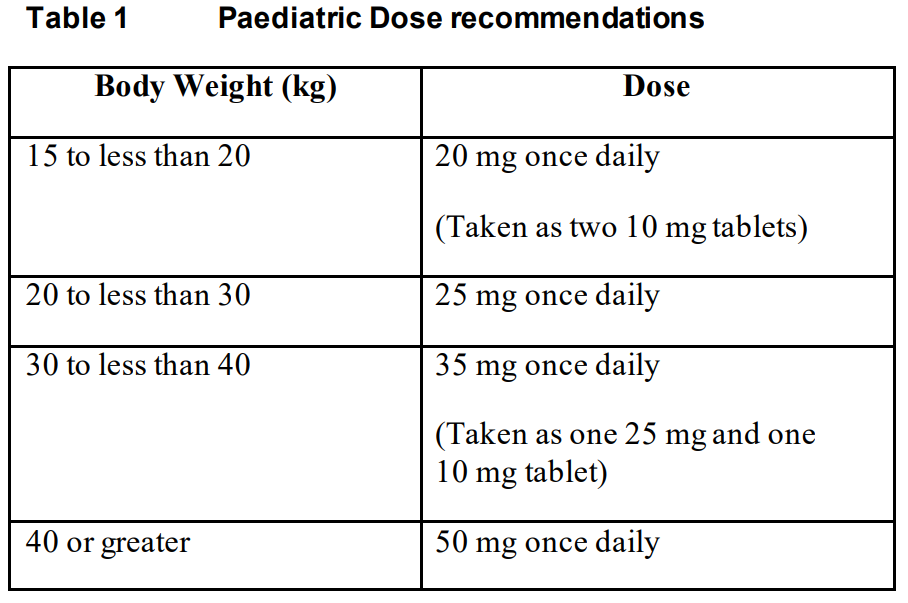 There are insufficient safety and efficacy data available to recommend a dose for _TIVICAY_ in children below age 6 or weighing less than 15 kg. There are insufficient data to recommend a dose for _TIVICAY_ in integrase inhibitor resistant children. **Elderly** There are limited data available on the use of _TIVICAY_ in patients aged 65 years and over. However, there is no evidence that elderly patients require a different dose than younger adult patients (see _Pharmacokinetics – Special Patient Populations_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Renal impairment** No dosage adjustment is required in patients with mild, moderate or severe (creatinine clearance (CrCl) <30 mL/min, not on dialysis) renal impairment. Limited data are available in subjects receiving dialysis, although differences in pharmacokinetics are not expected in this population (see _Pharmacokinetics – Special Patient Populations_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Hepatic impairment** No dosage adjustment is required in patients with mild or moderate hepatic impairment (Child-Pugh grade A or B). No data are available in patients with severe hepatic impairment (Child-Pugh grade C) (see _Pharmacokinetics – Special Patient Populations_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
ORAL
Medical Information
**Indications** _TIVICAY_ is indicated in combination with other antiretroviral agents for the treatment of human immunodeficiency virus type 1 (HIV-1) infection in adults and children aged 6 years and older and weighing at least 15 kg (see _Warnings and Precautions – Dual regimens_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The following should be considered prior to initiating treatment with _TIVICAY_: - Poor virologic response was observed in subjects treated with _TIVICAY_ 50 mg twice daily with an integrase strand transfer inhibitor (INI)-resistance Q148H/K/R substitution plus 2 or more additional INI-resistance substitutions, including, but not limited to L74I, E138A/K/T and G140A/C/S.
**Contraindications** _TIVICAY_ must not be administered concurrently with medicinal products with narrow therapeutic windows, that are substrates of organic cation transporter 2 (OCT2), including but not limited to dofetilide, pilsicainide or fampridine (also known as dalfampridine; see _Interactions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _TIVICAY_ is contraindicated in patients with known hypersensitivity to dolutegravir or to any of the excipients.
J05AX12
xj 05 ax 12
Manufacturer Information
GLAXOSMITHKLINE PTE LTD
GLAXO OPERATIONS UK LTD (TRADING AS GLAXO WELLCOME OPERATIONS)
Delpharm Poznań S.A.
Active Ingredients
Documents
Package Inserts
Tivicay Tablet PI.pdf
Approved: June 16, 2023
