Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
GRANULE, FOR SUSPENSION
**Dosage and Administration** Pharmaceutical Form: Dry, white to off-white, tutti-frutti flavoured granules for oral suspension. The usual course of therapy is seven days (range 5–10 days). For optimal absorption, _ZINNAT_ should be taken after food. **Dosage in adults:**  **Dosage in children:** There is no clinical trial data available on the use of _ZINNAT_ in children under the age of 3 months. 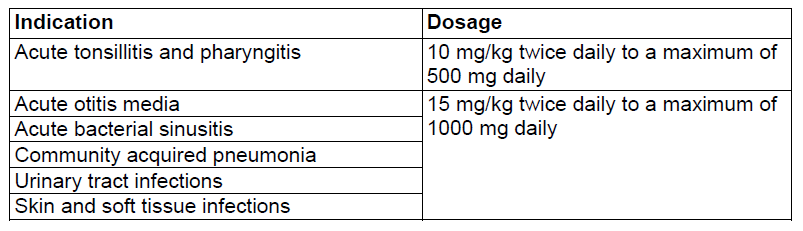 The following two tables serve as a guideline for simplified administration from measuring spoons (5ml) for the 125mg/5ml or the 250mg/5ml multidose suspension, and 125mg or 250mg single dose sachets. **10mg/kg dosage** 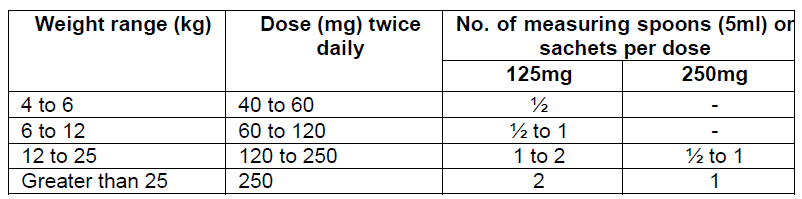 **15mg/kg dosage** 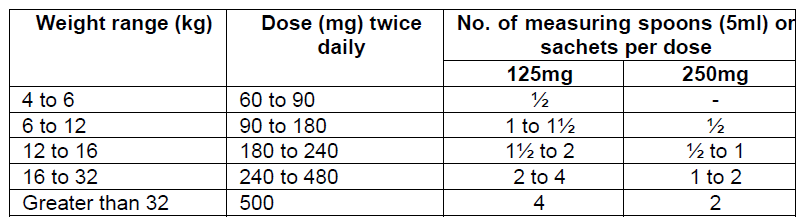 To enhance compliance and improve the dosing accuracy in very young children, a dosing syringe can be supplied with a multidose bottle containing 50ml of suspension. However, dosing in spoonfuls should be considered a more favourable option if the child is able to take the medication from the spoon. If required, the dosing syringe may also be used in older children (please refer to the dosing tables below). The recommended doses for the paediatric dosing syringe are expressed in ml or mg and according to body weight in the following tables. **10mg/kg/dose (Paediatric dosing syringe)** 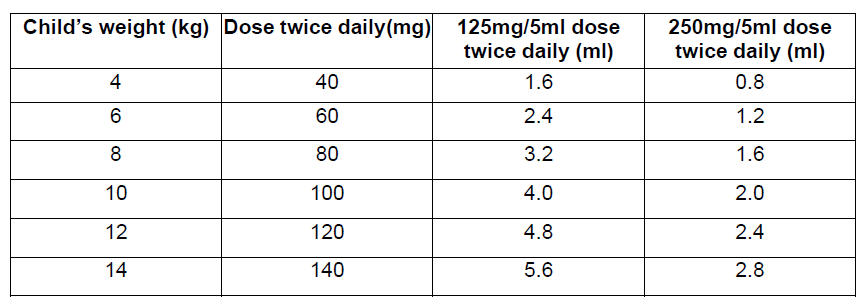 **15mg/kg/dose (Paediatric dosing syringe)** 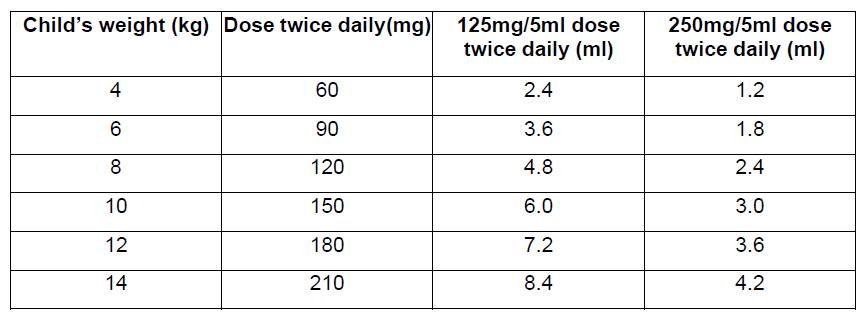 **Dosage in renal impairment:** Cefuroxime is primarily excreted by the kidneys. In patients with markedly impaired renal function, it is recommended that the dosage of cefuroxime be reduced to compensate for its slower excretion (see the table below). 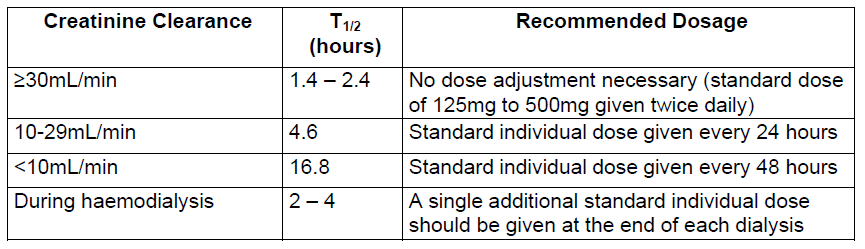
ORAL
Medical Information
**Indications** _ZINNAT_ is an oral prodrug of the bactericidal cephalosporin antibiotic cefuroxime, which is resistant to most β (beta)-lactamases and is active against a wide range of Gram-positive and Gram-negative organisms. It is indicated for the treatment of infections caused by susceptible bacteria. Susceptibility to _ZINNAT_ will vary with geography and time, and it should be used in accordance with local official antibiotic prescribing guidelines and local susceptibility data _(_ see _Pharmacological properties, Pharmacodynamics_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _)_. **Indications include:** Upper respiratory tract infections (for example: ear, nose and throat infections, such as otitis media, sinusitis, tonsillitis and pharyngitis). Lower respiratory tract infections (for example: pneumonia and acute exacerbations of chronic bronchitis). Genito-urinary tract infections (for example: pyelonephritis, cystitis and urethritis). Gonorrhoea, acute uncomplicated gonococcal urethritis and cervicitis. Skin and soft tissue infections (for example: furunculosis, pyoderma and impetigo).
**Contraindications** Patients with known hypersensitivity to cephalosporin antibiotics.
J01DC02
cefuroxime
Manufacturer Information
SANDOZ SINGAPORE PTE. LTD.
Glaxo Operations UK Limited (trading as Glaxo Wellcome Operations)
Active Ingredients
Documents
Package Inserts
Zinnat Suspension PI.pdf
Approved: November 4, 2022
