Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
POWDER, METERED
**4.2 Posology and method of administration** Foster NEXThaler is for inhalation use. ASTHMA Foster NEXThaler is not intended for the initial management of asthma. The dosage of Foster NEXThaler is individual and should be adjusted to the severity of the disease. This should be considered not only when treatment with combination products is initiated but also when the dose is adjusted. If an individual patient should require a combination of doses other than those available in the combination inhaler, appropriate doses of beta2-agonists and/or corticosteroids by individual inhalers should be prescribed. Because of its extrafine particle size distribution, dose adjustment is required when patients are transferred to Foster NEXThaler inhalation powder from a formulation with a non-extrafine particle size distribution. When switching patients from previous treatments, it should be considered that the recommended total daily dose of beclometasone dipropionate for Foster NEXThaler is lower than that for current beclometasone dipropionate-containing non-extrafine products and should be adjusted to the needs of the individual patient. However, patients who are transferred to Foster NEXThaler inhalation powder from **Foster** pressurised inhalation solution do not need dose adjustment. There are two treatment approaches: 1. **Maintenance therapy:** Foster NEXThaler is taken as regular maintenance treatment with a separate as needed rapid-acting bronchodilator. 2. **Maintenance and reliever therapy:** Foster NEXThaler is taken as regular maintenance treatment and as needed in response to asthma symptoms. **A. Maintenance therapy** Patients should be advised to have their separate rapid-acting bronchodilator available for rescue use at all times. _**Dose recommendations for adults 18 years and above:**_ One or two inhalations twice daily. The maximum daily dose is 4 inhalations daily. **B. Maintenance and reliever therapy** Patients take their daily maintenance dose of Foster NEXThaler and in addition take Foster NEXThaler as needed in response to asthma symptoms. Patients should be advised to always have Foster NEXThaler available for rescue use. Foster NEXThaler maintenance and reliever therapy should especially be considered for patients with: - not fully controlled asthma and in need of reliever medication - asthma exacerbations in the past requiring medical intervention Close monitoring for dose-related adverse effects is needed in patients who frequently take high numbers of Foster NEXThaler as-needed inhalations. **Dose recommendations for adults 18 years and above:** The recommended maintenance dose is 1 inhalation twice daily (one inhalation in the morning and one inhalation in the evening). Patients should take 1 additional inhalation as needed in response to symptoms. If symptoms persist after a few minutes, an additional inhalation should be taken. **The maximum daily dose is 8 inhalations.** Patients requiring frequent use of rescue inhalations daily should be strongly recommended to seek medical advice. Their asthma should be reassessed and their maintenance therapy should be reconsidered. _**Dose recommendations for children and adolescents under 18 years:**_ **The safety and efficacy of Foster NEXThaler in children and adolescents under 18 years of age have not yet been established. No data are available in children up to 11 years old. Currently available data in adolescents aged 12 – 17 years are summarised in sections 4.8 and 5.1 but no recommendation on a posology can be made** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ **.** Patients should be regularly reassessed by a doctor, so that the dosage of Foster NEXThaler remains optimal and is only changed on medical advice. The dose should be titrated to the lowest dose at which effective control of symptoms is maintained. When control of symptoms is maintained with the lowest recommended dosage, then the next step-down could include the inhaled corticosteroid alone. Patients should be advised to take Foster NEXThaler every day even when asymptomatic. **COPD** **Dose recommendations for adults 18 years and above:** Two inhalations twice daily. _**Special patient groups**_ There is no need to adjust the dose in elderly patients. There are no data available for use of Foster NEXThaler in patients with hepatic or renal impairment (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _**Method of administration**_ NEXThaler is a breath-operated inhaler. Moderate and severe asthmatic patients were shown to be able to produce sufficient inspiratory flow to trigger dose release from NEXThaler (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The delivery of Foster NEXThaler with Nexthaler is flow-independent in the range of inspiratory flow that this patient population can achieve through the inhaler. Correct use of the NEXThaler inhaler is essential in order for the treatment to be successful. The patient should be advised to read the Patient Information Leaflet carefully and follow the instructions for use as given in the leaflet. For the convenience of the prescriber these instructions are provided below. The number of doses shown in the window on the shell does not decrease on closing the cover if the patient has not inhaled through the inhaler. The patient should be instructed to only open the inhaler's cover when needed. In the event that the patient has opened the inhaler but not inhaled, and the cover is closed, the dose is moved back to the powder reservoir within the inhaler; the following dose can be safely inhaled. Patients should rinse their mouth or gargle with water or brush their teeth after inhaling (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **INSTRUCTIONS FOR USE OF NEXTHALER INHALER** 1. **Contents of the Package** For information on the Contents of the Package, see section 6.5 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. **If the package contents are not the same as described in section 6.5, return your inhaler to the person who supplied it and get a new one.** 2. **General Warnings & Precautions** - **Do not** remove the inhaler from the pouch if you do not intend to use it immediately. - Only use your inhaler as indicated. - Keep the cover closed until you need to take a dose from your inhaler. - When you are not using your inhaler, keep it in a clean and dry place. - **Do not** attempt to take your Nexthaler inhaler apart for any reason. 3. **Key features of your Nexthaler inhaler** 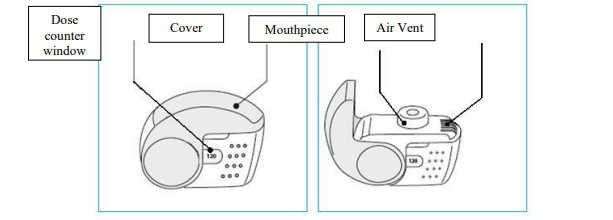 Taking a dose from your Nexthaler inhaler requires just three simple steps: Open, Inhale, Close. 4. **Before using a new Nexthaler inhaler** 1. **Open the pouch and take out your inhaler.** - **Do not** use your inhaler if the pouch is not sealed or it is damaged – return it to the person who supplied it and get a new one. - Use the label on the box to write down the date you open the pouch. 2. **Inspect your inhaler.** - If your inhaler looks broken or damaged, return it to the person who supplied it and get a new one. 3. **Check the Dose Counter Window. If your inhaler is brand new you will see “120” in the Dose Counter Window.** - **Do not** use a new inhaler if the number shown is less than “120” – return it to the person who supplied it and get a new one. 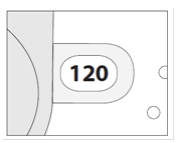 5. **How to use your Nexthaler inhaler** - If you are not sure you are receiving your dose correctly, contact your pharmacist or doctor. - If you are not sure the dose counter has gone down by one after inhalation, wait until your next scheduled dose and take this as normal. Do not take an extra dose. **E.1. Open** 1. **Hold your inhaler firmly in the upright position.** 2. **Check the number of doses left: any number between “1” and “120” shows that there are doses left.** - If the Dose Counter Window shows “0” there are no doses left – dispose of your inhaler and get a new one. 3. **Open the cover fully.** 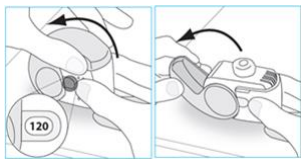 4. **Before inhaling breathe out as far as is comfortable.** - **Do not** breathe out through your inhaler. **E.2. Inhale** **Whenever possible, stand or sit in an upright position when inhaling.** 1. **Lift your inhaler up, bring it to your mouth and place your lips around the mouthpiece.** - **Do not** cover the air vent when holding your inhaler. - **Do not** inhale through the air vent. 2. **Take a quick and deep breath through your mouth.** - You may notice a taste when you take your dose. - You may hear or feel a click when you take your dose. - **Do not** inhale through your nose. - **Do not** remove your inhaler from your lips during the inhalation. 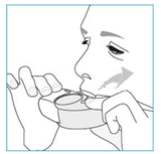 3. **Remove your inhaler from your mouth.** 4. **Hold your breath for 5 to 10 seconds or as long as is comfortable.** 5. **Breathe out slowly.** - **Do not** breathe out through your inhaler. **E.3. Close** 1. **Move your inhaler back to the upright position and close the cover fully.** 2. **Check that the dose counter has gone down by one.** 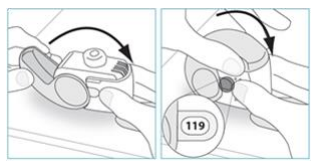 3. **If you need to take another dose, repeat steps E.1 to E.3.** 6. **Cleaning** - Normally, it is not necessary to clean your inhaler. - If necessary, you may clean your inhaler after use with a dry cloth or tissue. - **Do not** clean your inhaler with water or other liquids. Keep it dry. 7. **Storage and Disposal** For information on the Storage and Disposal, see section 6.4 and 6.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
RESPIRATORY (INHALATION)
Medical Information
**4.1 Therapeutic indications** **ASTHMA** Foster NEXThaler is indicated in the regular treatment of asthma where use of a combination product (inhaled corticosteroid and long-acting beta2-agonist) is appropriate: - patients not adequately controlled with inhaled corticosteroids and 'as needed' inhaled short-acting beta2-agonist or - patients already adequately controlled on both inhaled corticosteroids and long-acting beta2-agonists. Foster NEXThaler is indicated for adult patients. **COPD** Symptomatic treatment of patients with severe COPD (FEV1 < 50% predicted normal) and a history of repeated exacerbations, who have significant symptoms despite regular therapy with long-acting bronchodilators.
**4.3 Contraindications** Hypersensitivity to beclometasone dipropionate, formoterol fumarate dihydrate or to any of the excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
R03AK08
formoterol and beclometasone
Manufacturer Information
ORIENT EUROPHARMA PTE LTD
Chiesi Farmaceutici SpA
Active Ingredients
Documents
Package Inserts
Foster NEXThaler 100-6 micrograms per dose inhalation powder PI.pdf
Approved: August 20, 2020
