Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INFUSION, SOLUTION
**DOSAGE REGIMEN AND ADMINISTRATION** **The Zometa 4 mg/5 mL concentrate for solution for infusion** should be further diluted with 100 mL 0.9% w/v sodium chloride or 5% w/v glucose solution before infusion (see section INSTRUCTIONS FOR USE AND HANDLING – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The final Zometa solution for infusion, should be given as an intravenous infusion of no less than 15 minutes. **The Zometa 4 mg/100 mL solution for infusion** is a “ready to use” presentation and must not be further diluted or mixed with other infusion solutions except for patients with renal impairment. It should be administered as a single intravenous solution in a separate infusion line in no less than 15 minutes. **Dosage regimen** **Treatment of bone metastases and treatment of osteolytic lesions, in conjunction with standard antineoplastic therapy** In adults and elderly patients, the recommended Zometa dose is a 4 mg infusion given every 3 to 4 weeks. Patients should also be administered an oral calcium supplement of 500 mg and 400 international units vitamin D daily. **Treatment of hypercalcemia of malignancy (HCM)** In adult and elderly patients, the recommended Zometa dose in hypercalcemia (albumin-corrected serum calcium ≥ 12.0 mg/dL or 3.0 mmol/L) is a single 4 mg infusion. Patients must be maintained well hydrated prior to and following administration of Zometa. **Treatment of patients with renal impairment** **Patients with hypercalcemia with malignancy (HCM)** Zometa treatment in adult patients with hypercalcemia of malignancy (HCM) who also have severe renal impairment should be considered only after evaluating the risks and benefit of treatment. In the clinical studies, patients with serum creatinine >400 micromol/L or >4.5 mg/dL were excluded. No dose adjustment is necessary in HCM patients with serum creatinine < 400 micromol/L or < 4.5 mg/dL (see section SPECIAL WARNINGS AND PRECAUTIONS FOR USE – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Treatment of bone metastases and treatment of osteolytic lesions, in conjunction with standard antineoplastic therapy.** When initiating treatment with Zometa, serum creatinine levels and creatinine clearance (CLcr) should be determined. CLcr is calculated from serum creatinine levels using the Cockcroft-Gault formula. Zometa is not recommended for patients presenting with severe renal impairment prior to initiation of therapy, which is defined for this population as CLcr < 30 mL/min. In clinical trials with Zometa, patients with serum creatinine > 265 micromol/L or > 3.0 mg/dL were excluded. In patients with bone metastases presenting with mild to moderate renal impairment prior to initiation of therapy, which is defined for this population as CrCl 30 to 60 mL/min, the following Zometa dose is recommended (see also section SPECIAL WARNINGS AND PRECAUTIONS FOR USE – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_): 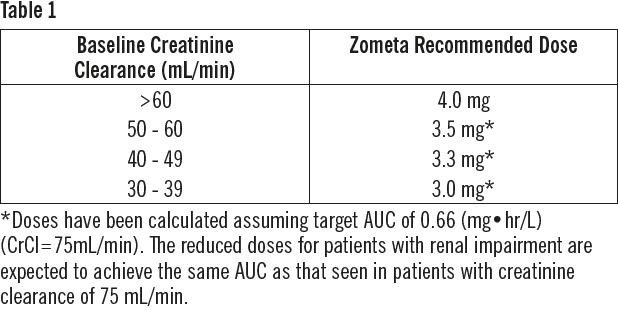 Following initiation of therapy, serum creatinine should be measured prior to each dose of Zometa and treatment should be withheld if renal function has deteriorated. In the clinical trials, renal deterioration was defined as follows: - For patients with normal baseline serum creatinine (< 1.4 mg/dL), an increase of ≥ 0.5 mg/dL; - For patients with an abnormal baseline creatinine (> 1.4 mg/dL), an increase of ≥ 1.0 mg/dL. In the clinical studies, Zometa treatment was resumed only when the creatinine level returned to within 10% of the baseline value (see section WARNINGS AND PRECAUTIONS – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Zometa should be resumed at the same dose as that prior to treatment interruption. **Method of administration** Zometa must only be administered to patients by healthcare professionals experienced in the administration of intravenous bisphosphonates. Zometa must not be mixed with calcium or other divalent cation-containing infusion solutions, such as Lactated Ringer’s solution, and should be administered as a single intravenous solution in a line separate from all other drugs in no less than 15 minutes. Patients must be maintained in a well hydrated state prior to and following administration of Zometa. **Preparation of reduced Zometa doses** In patients with mild to moderate renal impairment, which is defined as CLcr 30 to 60 mL/min, reduced Zometa dosages are recommended, except in patients with HCM (see section Dosage regimen sub-section). To prepare reduced doses of Zometa 4 mg/5 mL concentrate, withdraw an appropriate volume of the liquid concentrate needed, as follows: 4.4 mL for 3.5 mg dose 4.1 mL for 3.3 mg dose 3.8 mL for 3.0 mg dose For information on the reconstitution and dilution of Zometa (see section INSTRUCTIONS FOR USE AND HANDLING – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The withdrawn amount of the concentrate must be diluted in 100 mL of sterile 0.9% w/v sodium chloride solution or 5% w/v glucose solution. The dose must be given as a single intravenous infusion of no less than 15 minutes. To prepare reduced doses of Zometa 4 mg/100 mL solution for infusion, remove the corresponding volume of Zometa solution as indicated below and replace it with an equal volume of sterile 0.9% w/v sodium chloride solution or 5% w/v glucose solution. 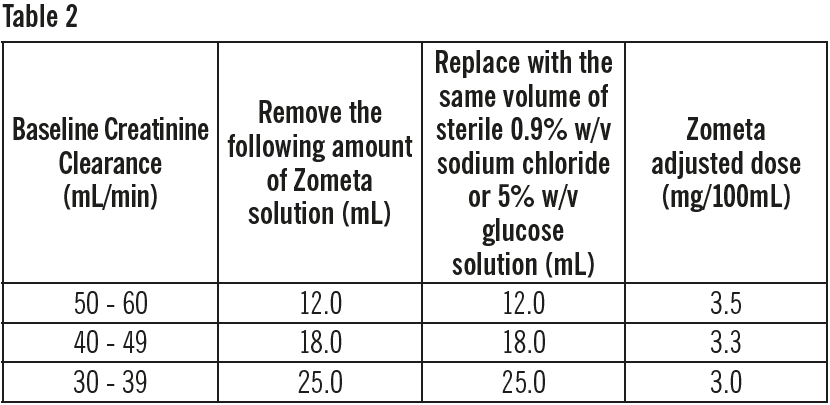
INTRAVENOUS
Medical Information
**INDICATIONS** - Treatment of osteolytic, osteoblastic, and mixed bone metastases of solid tumours and osteolytic lesions of multiple myeloma, in conjunction with standard antineoplastic therapy. - Treatment of hypercalcemia of malignancy (HCM).
**CONTRAINDICATIONS** - Hypersensitivity to zoledronic acid or other bisphosphonates or any of the excipients in the formulation of Zometa. - Pregnancy and breast-feeding women (see section PREGNANCY, LACTATION, FEMALES AND MALES OF REPRODUCTIVE POTENTIAL – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
M05BA08
zoledronic acid
Manufacturer Information
SciGen Pte Ltd.
Novartis Pharma Stein AG
Fresenius Kabi Austria GmbH
FISIOPHARMA S.R.L.
Active Ingredients
Documents
Package Inserts
Zometa PI.pdf
Approved: September 23, 2020
