Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
SOLUTION
**Dosage and Administration** **Dosage** **_Schizophrenia_** **_Switching from other antipsychotics_** When medically appropriate, gradual discontinuation of the previous treatment while RISPERDAL® therapy is initiated is recommended. Also, if medically appropriate, when switching patients from depot antipsychotics, initiate RISPERDAL® therapy in place of the next scheduled injection. The need for continuing existing anti-Parkinson medications should be re-evaluated periodically. **_Adults_** RISPERDAL® may be given once daily or twice daily. Patients should start with 2 mg/day RISPERDAL®. The dosage may be increased on the second day to 4 mg. From then on the dosage can be maintained unchanged, or further individualized, if needed. Most patients will benefit from daily doses between 4 and 6 mg. In some patients, a slower titration phase and a lower starting and maintenance dose may be appropriate. Doses above 10 mg/day have not been shown to be superior in efficacy to lower doses and may cause extrapyramidal symptoms. Since the safety of doses above 16 mg/day has not been evaluated, doses above this level should not be used. A benzodiazepine may be added to RISPERDAL® when additional sedation is required. **_Special populations_** **_Elderly_** A starting dose of 0.5 mg twice daily is recommended. This dosage can be individually adjusted with 0.5 mg twice daily increments to 1 to 2 mg twice daily. **_Children_** Experience in schizophrenia is lacking in children less than 15 years of age. **_Renal and liver disease_** A starting dose of 0.5 mg twice daily is recommended. This dose can be individually adjusted with 0.5 mg twice daily increments to 1 to 2 mg twice daily. **_Bipolar mania_** **_Adults_** RISPERDAL® should be administered on a once daily schedule, starting with 2 or 3 mg. Dosage adjustments, if indicated, should occur at intervals of not less than 24 hours and in dosage increments of 1 mg per day. Efficacy was demonstrated in flexible doses over a range of 1 to 6mg per day. A dosing range between 2–6 mg per day is recommended. The physician who elects to use RISPERDAL® for periods extending beyond 12 weeks should periodically re-evaluate the long-term usefulness of the drug for the individual patient. As with all symptomatic treatments, the continued use or RISPERDAL® must be evaluated and justified on an ongoing basis. **_Children_** Experience is lacking in bipolar mania in children and adolescents less than 18 years of age. **_Aggression in patients with Dementia of the Alzheimer type_** A starting dose of 0.25 mg b.i.d. is recommended. This dosage can be individually adjusted by increments of 0.25 mg b.i.d., not more frequently than every other day, if needed. The optimum dose is 0.5 mg b.i.d. for most patients. Some patients, however, may benefit from doses up to 1 mg b.i.d. There is no data to support treatment beyond 12 weeks in patients with moderate to severe dementia of the Alzheimer type with agitation, aggression or psychotic symptoms. Once patients have reached their target dose, a once daily dosing regimen can be considered. As with all symptomatic treatments, the continued use or RISPERDAL® must be evaluated and justified on an ongoing basis. **_Conduct and other disruptive behavior disorders (5–18 years of age)_** For patients ≥ 50 kg, a starting dose of 0.5 mg once daily is recommended. This dosage can be individually adjusted by increments of 0.5 mg once daily not more frequently than every other day, if needed. The optimum dose is 1 mg once daily for most patients. Some patients, however, may benefit from 0.5 mg once daily while others may require 1.5 mg once daily. For patients < 50 kg, a starting dose of 0.25 mg once daily is recommended. This dosage can be individually adjusted by increments of 0.25 mg once daily not more frequently than every other day, if needed. The optimum dose is 0.5 mg once daily for most patients. Some patients, however, may benefit from 0.25 mg once daily while others may require 0.75 mg once daily. As with all symptomatic treatments, the continued use of RISPERDAL® must be evaluated and justified on an ongoing basis. Experience is lacking in children less than 5 years of age. **_Autism_** **_Pediatrics (5–17 years of age)_** The dosage of RISPERDAL® should be individualized according to the needs and response of the patient. Dosing should be initiated at 0.25 mg per day for patients <20 kg and 0.5 mg per day for patients ≥20 kg. On Day 4, the dose may be increased by 0.25 mg for patients <20 kg and 0.5 mg for patients ≥20 kg. This dose should be maintained and response should be assessed at approximately Day 14. Only in patients not achieving sufficient clinical response should additional dose increases be considered. Dose increases may proceed at ≥2-week intervals in increments of 0.25 mg for patients <20 kg or 0.5 mg for patients ≥20 kg. In clinical studies, the maximum dose studied did not exceed a total daily dose of 1.5 mg in patients <20 kg, 2.5 mg in patients ≥20 kg, or 3.5 mg in patients >45 kg. Doses below 0.25mg/day were not effective in clinical studies. 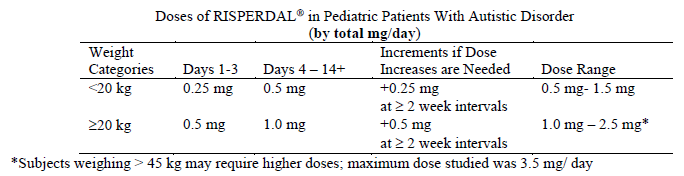 For prescribers preferring to dose on a mg/kg/day basis the following guidance is provided.  RISPERDAL® can be administered once daily or twice daily. Patients experiencing somnolence may benefit from a switch in dosing from once daily to either once daily at bedtime or twice daily. Once sufficient clinical response has been achieved and maintained, consideration may be given to gradually lowering the dose to achieve the optimal balance of efficacy and safety. Experience is lacking in children less than 5 years and limited in autistic adolescents. Effectiveness for more than 8 weeks has not been systematically evaluated in double-blind, parallel-controlled clinical trials. Therefore, the physician who elects to use RISPERDAL® for the treatment of behavioral disorders associated with autism (e.g. irritability, social withdrawal, stereotypic behaviour, hyperactivity and inappropriated speech) in children and adolescents for extended periods should periodically re-evaluate the long term risks and benefits of the drug for the individual patient. **_Renal and hepatic impairment_** Patients with renal impairment have less ability to eliminate the active antipsychotic fraction than normal adults. Patients with impaired hepatic function have increases in plasma concentration of the free fraction of risperidone. Irrespective of the indication, starting and consecutive dosing should be halved, and dose titration should be slower for patients with renal or hepatic impairment. RISPERDAL® should be used with caution in these groups of patients. **Administration** RISPERDAL® may be given as oral tablets or oral solution.
ORAL
Medical Information
**Indications** RISPERDAL® is indicated for the treatment of a broad range of patients with schizophrenia, including first episode psychoses, acute schizophrenic exacerbations, chronic schizophrenia, and other psychotic conditions, in which positive symptoms (such as hallucinations, delusions, thought disturbances, hostility, suspiciousness), and/or negative symptoms (such as blunted affect, emotional and social withdrawal, poverty of speech) are prominent. RISPERDAL® alleviates affective symptoms (such as depression, guilt feelings, anxiety) associated with schizophrenia. RISPERDAL® is also effective in maintaining the clinical improvement during continuation therapy in patients who have shown an initial treatment response. RISPERDAL® is indicated for the short-term treatment of persistent aggression in patients with moderate to severe dementia of the Alzheimer’s type unresponsive to non-pharmacological approaches and when there is a risk of harm to self or others. RISPERDAL® is indicated for the treatment of behavioural disorders associated with autism (e.g. irritability, social withdrawal, stereotypic behaviour, hyperactivity and inappropriate speech) in children and adolescents. RISPERDAL® is also indicated for bipolar mania. **_Adjunctive therapy:_** RISPERDAL® is indicated as adjunctive therapy to mood stabilizers in the treatment of manic episodes associated with bipolar disorders. These episodes are characterized by symptoms such as elevated, expansive or irritable mood, inflated self-esteem, decreased need for sleep, pressured speech, racing thoughts, distractibility, or poor judgment, including disruptive or aggressive behaviors. **_Monotherapy:_** RISPERDAL® is indicated in the treatment of acute manic episodes associated with bipolar 1 disorder. The effectiveness of RISPERDAL® for more than 12 weeks of treatment of an acute episode, and for the prevention of new manic episodes has not been established. RISPERDAL® is indicated in the treatment of conduct and other disruptive behavior disorders in children (over 5 years), adolescents and adults with subaverage intellectual functioning or mental retardation in whom destructive behaviors (e.g. aggression, impulsivity and self-injurious behaviors) are prominent. As with all symptomatic treatments, the continued use of RISPERDAL® must be evaluated and justified on an ongoing basis.
**Contraindications** RISPERDAL® is contraindicated in patients with a known hypersensitivity to the product.
N05AX08
risperidone
Manufacturer Information
JOHNSON & JOHNSON INTERNATIONAL (SINGAPORE) PTE. LTD.
JANSSEN PHARMACEUTICA NV
Active Ingredients
Documents
Package Inserts
Risperdal Oral Solution and Tablet PI.pdf
Approved: May 8, 2023
