Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, SUGAR COATED
**4.2 Dosage and method of administration** **4.2.1 Method of administration** Oral use. **4.2.2 Dosage regimen** Hormonal contraception should be stopped when HRT is started and the patient should be advised to take non-hormonal contraceptive precautions, if required. - **How to start Progyluton** If the patient is still menstruating, treatment should begin on the 5th day of the cycle (1st day of menstrual bleeding = 1st day of the cycle). Patients with amenorrhea or very infrequent periods or who are post-menopausal may start at any time, provided pregnancy has been excluded (see section 4.6, “Pregnancy and lactation” – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). - Dosage One white tablet is taken daily for the first 11 days, followed by one light brown tablet daily for 10 days. Following the 21 days of tablet-taking there will be a tablet-free interval of 7 days. - Administration Each pack covers 21 days of treatment. A new pack of Progyluton should be started after the 7-day tablet-free interval on the same day of the week as the previous one. The tablets are to be swallowed whole with some liquid. The tablets should preferably be taken at the same time every day. Estrogen with or without progestogens should be prescribed at the lowest effective doses and for the shortest duration consistent with treatment goals and risks for the individual women. The Progyluton pack contains a self-adhesive sticker marked with the days of the week. Choose the strip that starts with the day the patient begin tablet taking. For example, if the patient starts the tablets on a Wednesday, use the strip that starts with “Wed”. The strip should be stuck along the top of the blister-pack on the side, where the tablets are visible, so that the first day is above the first tablet of the row (see Figure). Each tablet is thus marked with the corresponding day of the week and one can see at a glance whether the tablet for that day has been taken or not. Follow the direction of the arrows from left to right until all 21 tablets have been taken. It does not matter at what time of the day the patient takes her tablet, but once she has selected a particular time, she should keep to it every day. If she forgets to take a tablet at the usual time she may take it within the following 12 to 24 hours. If the treatment is discontinued for longer, irregular bleeding may occur. 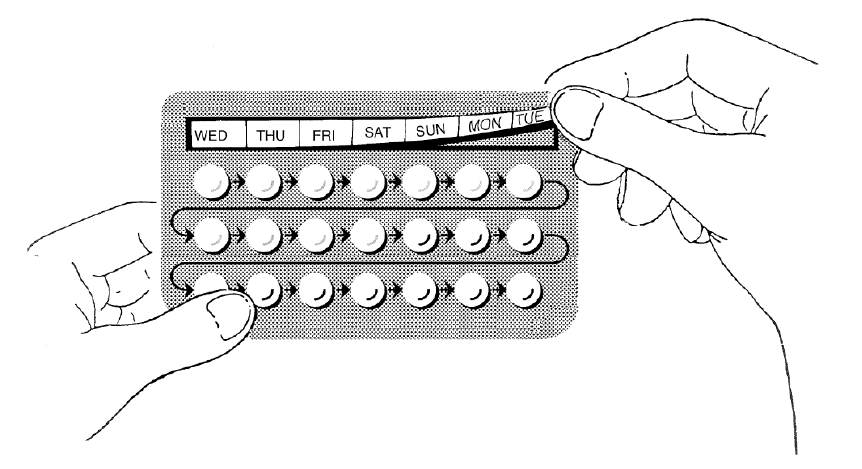 Bleeding usually occurs during the tablet-free interval of 7 days a few days after the last tablet was taken. - Missed tablets In case a tablet is forgotten, it should be taken as soon as possible. If more than 24 hours have elapsed, no extra tablet needs to be taken. If several tablets are forgotten, bleeding may occur. Bleeding usually occurs during the tablet-free interval of 7 days within a few days after the last tablet was taken. **4.2.3 Additional information on special populations** **4.2.3.1 Pediatric patients** Progyluton is not indicated for use in children and adolescents. **4.2.3.2 Geriatric patients** There are no data suggesting a need for dosage adjustment in elderly patients. **4.2.3.3 Patients with hepatic impairment** Progyluton has not been specifically studied in patients with hepatic impairment. Progyluton is contraindicated in women with presence or history of liver tumors and with severe hepatic disease (see section, “Contraindications”). For women with impaired liver function, close supervision is needed and in case of deterioration of markers of liver function, use of HRT should be stopped (see section ‘Special warnings and precautions for use’ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **4.3.3.4 Patients with renal impairment** Progyluton has not been specifically studied in renally impaired patients. Estrogen may cause fluid retention, and therefore patients with cardiac or renal dysfunction should be carefully observed.
ORAL
Medical Information
**4.1 Indications** Hormone replacement therapy (HRT) for the treatment of signs and symptoms of estrogen deficiency due to menopause or hypogonadism, castration or primary ovarian failure in women with an intact uterus. Prevention of postmenopausal osteoporosis. Control of irregular menstrual cycles. Treatment of primary or secondary amenorrhea.
**4.3 Contraindication** Hormone replacement therapy (HRT) should not be started in the presence of any of the conditions listed below. Should any of the conditions appear during HRT use, the product should be stopped immediately. - Pregnancy and lactation - Undiagnosed vaginal bleeding - Known or suspected cancer of the breast - Known or suspected premalignant conditions or malignancies, if sex steroid-influenced - Presence or history of liver tumours (benign or malignant) - Severe hepatic disease - Acute arterial thromboembolism (e.g. myocardial infarction, stroke) - Active deep venous thrombosis, thromboembolic disorders, or a documented history of these conditions - A high risk of venous or arterial thrombosis - Severe hypertriglyceridemia - Known hypersensitivity to any of the components of Progyluton
G03AA06
norgestrel and ethinylestradiol
Manufacturer Information
BAYER (SOUTH EAST ASIA) PTE LTD
BAYER WEIMAR GMBH UND CO. KG
Active Ingredients
Documents
Package Inserts
1.4.3_Proposed Pristine PI_Progyluton_CCDS 15+16 & HSA HRTs Safety Update_24 Nov 2021.pdf
Approved: April 19, 2022
