Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET
**DOSAGE REGIMEN AND ADMINISTRATION** **Dosage regimen** The dosage of Ritalin should be individualized according to the patient’s clinical needs and responses. In the treatment of ADHD, an attempt should be made to time administration to coincide with the periods of greatest academic, behavioral, or social stress. Ritalin should be started at a low dose, with increments at weekly intervals. Daily doses above 60 mg are not recommended for the treatment of narcolepsy, or for the treatment of ADHD in children. Daily doses above 80 mg are not recommended for the treatment of ADHD in adults (Ritalin LA only). If symptoms do not improve after dose titration over a period of one month, the drug should be discontinued. If symptoms worsen or other adverse effects occur, the dosage should be reduced or, if necessary, the drug discontinued. If the effect of the drug wears off too early in the evening, disturbed behaviour and/or inability to go to sleep may recur. A small evening dose of Ritalin may help to solve this problem. **Pre-treatment screening** Before initiating Ritalin treatment, patients should be assessed for pre-existing cardiovascular and psychiatric disorders and a family history of sudden death, ventricular arrhythmia and psychiatric disorders. Weight and height should also be measured before treatment and documented on a growth chart (see sections CONTRAINDICATIONS and WARNINGS AND PRECAUTIONS – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Periodic assessment of the treatment in ADHD** Drug treatment does not need to be indefinite. Physicians should periodically re-evaluate the treatment with trial periods off medication to assess the patient’s functioning without pharmacotherapy. Improvement may be sustained when the drug is either temporarily or permanently discontinued. When used in children with ADHD, treatment can usually be discontinued during or after puberty. **ADHD** **Children and adolescents (6 years and over)** Tablets: Start with 5 mg once or twice daily (e.g. at breakfast and lunch) with weekly increments of 5 to 10 mg. The total daily dosage should be administered in divided doses. LA capsules are for oral administration once daily in the morning. The recommended starting dose of Ritalin LA is 20 mg. A maximum dose of 60 mg should not be exceeded. **Adults** Only the Ritalin LA formulation should be used for the treatment of ADHD in adults. Ritalin LA is administered once daily. Patients new to methylphenidate (see section CLINICAL PHARMACOLOGY – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_): The recommended starting dose of Ritalin LA in patients who are not currently taking methylphenidate is 20 mg once daily. Patients currently using methylphenidate: Treatment may be continued with the same daily dose. If the patient was previously treated with an immediate release formulation, a switch to an appropriate recommended dose of Ritalin LA should be made (see below subsection switching patient’s treatment to Ritalin LA). A maximum daily dose of 80 mg should not be exceeded. No difference in dosing is recommended between male and female adult patients (see section CLINICAL STUDIES – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Switching patient’s treatment to Ritalin LA** The recommended dose of Ritalin LA should be equal to the total daily dose of the immediate-release formulation not exceeding a total dose of 60 mg in children and 80 mg in adults. Examples involving switch from the immediate-release formulation or the sustained-release formulation are provided below. 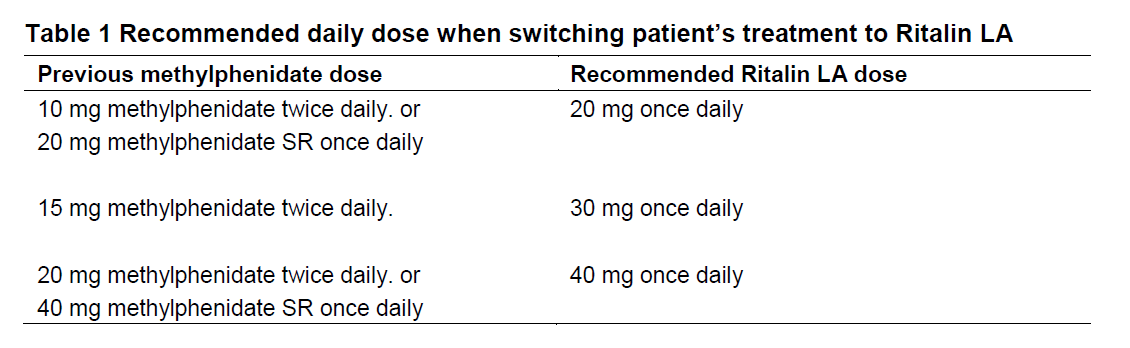 For other methylphenidate regimens, clinical judgment should be used when selecting the starting dose. Ritalin LA dosage may be adjusted at weekly intervals in 10 mg increments for children and in 20 mg increments for adults. **Narcolepsy** Only the Ritalin formulation is approved in the treatment of narcolepsy in adults. Tablets: The average daily dose is 20 to 30 mg, given in 2 to 3 divided doses. Some patients may require 40 to 60 mg daily, while for others, 10 to 15 mg daily will be adequate. Patients who are unable to sleep if medication is taken late in the day should take the last dose before 6 p.m. A maximum daily dose of 60 mg should not be exceeded. **Special populations** **Renal impairment** No studies have been performed in renally impaired patients (see section CLINICAL PHARMACOLOGY – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Hepatic impairment** No studies have been performed in hepatically impaired patients (see section CLINICAL PHARMACOLOGY – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Geriatric patients (65 years and above)** No studies have been performed in patients over 60 years of age (see section CLINICAL PHARMACOLOGY – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Method of administration** **General recommendations** **Tablets** can be taken with or without food (see section CLINICAL PHARMACOLOGY – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). LA capsules and/or their contents should not be crushed, chewed, or divided. Ritalin LA capsules may be administered with or without food. They may be swallowed whole or alternatively may be administered by sprinkling the contents over a small amount of food (see specific instructions below). **Ritalin LA administration by sprinkling capsule contents on food** The capsules may be carefully opened and the beads sprinkled over soft food (e.g. apple sauce). The food should not be warm because this could affect the modified-release properties of this formulation. The mixture of drug and food should be consumed immediately in its entirety. The drug and food mixture should not be stored for future use. Ritalin LA, administered as a single dose, provides comparable overall exposure (AUC) of methylphenidate to the same total dose of Ritalin administered twice daily.
ORAL
Medical Information
**INDICATIONS** **Attention-Deficit/Hyperactivity Disorder (ADHD, DSM-IV)** Ritalin is indicated in the treatment of Attention-Deficit/Hyperactivity Disorder (ADHD) in children aged 6 years or older. Ritalin LA is indicated in the treatment of ADHD in children aged 6 years or older, and in adults. ADHD was previously known as attention-deficit disorder or minimal brain dysfunction. Other terms used to describe this behavioral syndrome include: hyperkinetic disorder, minimal brain damage, minimal cerebral dysfunction, minor cerebral dysfunction and psycho-organic syndrome of patients. Ritalin is indicated as part of a comprehensive treatment program which typically includes psychological, educational, and social measures and is aimed at stabilizing patients with a behavioral syndrome characterised by moderate to severe distractibility, short attention span, hyperactivity, emotional lability, and impulsivity. The diagnosis should be made according to DSM-IV criteria or the guidelines in ICD-10. Non-localizing (soft) neurological signs, learning disability, and abnormal EEG may or may not be present, and a diagnosis of central nervous system dysfunction may or may not be warranted. **Special Diagnostic Considerations for ADHD in children** The specific etiology of this syndrome is unknown, and there is no single diagnostic test. Proper diagnosis requires medical and neuropsychological, educational, and social investigation. Characteristics commonly reported include: history of short attention span, distractibility, emotional lability, impulsivity, and moderate-to-severe hyperactivity, minor neurological signs, and abnormal EEG. Learning may or may not be impaired. The diagnosis must be based upon a complete history and evaluation of the child and not solely on the presence of one or more of these characteristics. Drug treatment is not indicated in all children with this syndrome. Stimulants are not indicated in children with symptoms secondary to environmental factors (child abuse in particular) and/or primary psychiatric disorder, including psychosis. Appropriate educational placement is essential, and psychosocial intervention is generally necessary. Where remedial measures alone prove insufficient, the decision to prescribe a stimulant must be based on rigorous assessment of the severity of the child’s symptoms. **Special Diagnostic Considerations for ADHD in adults** The specific etiology of this syndrome is unknown, and there is no single diagnostic test. Adults with ADHD have symptom patterns characterized by shifting activities, becoming bored easily, restlessness, impatience, and inattentiveness. Symptoms such as hyperactivity tend to diminish with increasing age possibly due to adaptation, neurodevelopment and self-medication. Inattentive symptoms are more prominent and have a greater impact on adults with ADHD. Diagnosis in adults should include a structured patient interview to determine current symptoms. The preexistence of childhood ADHD is to be determined retrospectively. Diagnosis should not be made solely on the presence of one or more symptoms. The decision to use a stimulant in adults must be based on a very thorough assessment of the severity and chronicity of the symptoms and their impact on the daily life of the patient. **Narcolepsy (Ritalin only)** Ritalin is indicated for the treatment of narcolepsy in adults. Symptoms include daytime sleepiness, inappropriate sleep episodes, and sudden loss of voluntary muscle tone.
**CONTRAINDICATIONS** - Hypersensitivity to methylphenidate or to any of the excipients. - Anxiety, tension. - Agitation. - Hyperthyroidism. - Pre-existing cardiovascular disorders including severe hypertension, angina, arterial occlusive disease; heart failure, hemodynamically significant congenital heart disease, cardiomyopathies, myocardial infarction, potentially life-threatening arrhythmias and channelopathies (disorders caused by the dysfunction of ion channels). - During treatment with monoamine oxidase (MAO) inhibitors, or within a minimum of 2 weeks of discontinuing those drugs, due to risk of hypertensive crisis (see section INTERACTIONS – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). - Glaucoma. - Phaeochromocytoma. - Diagnosis or family history of Tourette's syndrome.
N06BA04
methylphenidate
Manufacturer Information
NOVARTIS (SINGAPORE) PTE LTD
Siegfried Barbera S.L
Active Ingredients
Documents
Package Inserts
Ritalin PI.pdf
Approved: April 26, 2022
