Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION
**Posology and method of administration:** Posology: The dose of Ertapenem in patients 13 years of age and older is 1 gram (g) given once a day. The dose of Ertapenem in patients 3 months to 12 years of age is 15 mg/kg twice daily (not to exceed 1 g/day). Ertapenem is not recommended in children under 3 months of age, as no data are available. Method of administration: Ertapenem may be administered by intravenous infusion for up to 14 days or intramuscular injection for up to 7 days. When administered intravenously, Ertapenem should be infused over a period of 30 minutes. Intramuscular administration of Ertapenem may be used as an alternative to intravenous administration in the treatment of those infections for which intramuscular therapy is appropriate. DO NOT MIX OR CO-INFUSE ERTAPENEM WITH OTHER MEDICATIONS. DO NOT USE DILUENTS CONTAINING DEXTROSE (α-D-GLUCOSE). 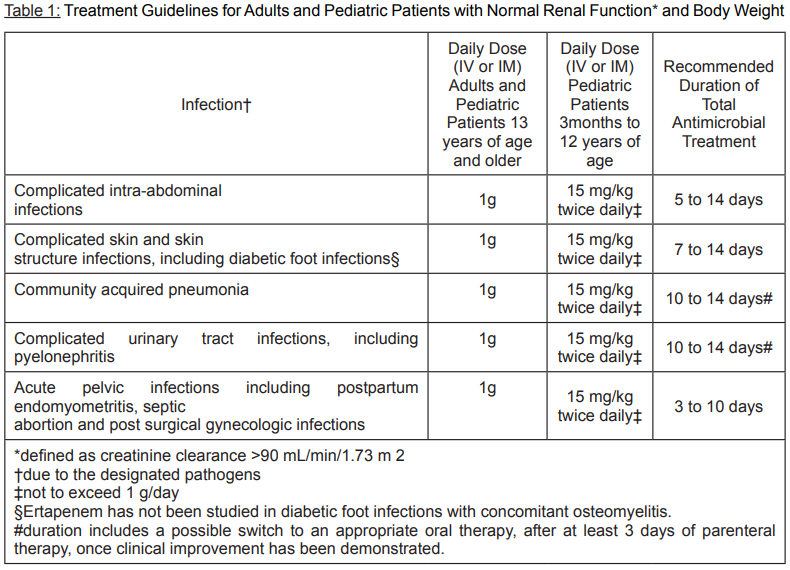 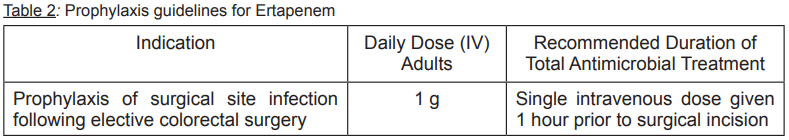 **Patients with Renal Insufficiency:** Ertapenem may be used for the treatment of infections in adult patients with renal insufficiency. In patients whose creatinine clearance is >30 mL/min/1.73 m2, no dosage adjustment is necessary. Adult patients with advanced renal insufficiency (creatinine clearance ≤30mL/min/1.73 m2) including those on haemodialysis should receive 500 mg daily. There are no data in pediatric patients with renal insufficiency. **Patients on Hemodialysis:** When adult patients on hemodialysis are given the recommended daily dose of 500 mg of Ertapenem within 6 hours prior to hemodialysis, a supplementary dose of 150 mg is recommended following the hemodialysis session. If ERTAPENEM is given at least 6 hours prior to hemodialysis, no supplementary dose is needed. There are no data in patients undergoing peritoneal dialysis or hemofiltration. There are no data in pediatric patients on hemodialysis. When only the serum creatinine is available, the following formula may be used to estimate creatinine clearance. The serum creatinine should represent a steady state of renal function. 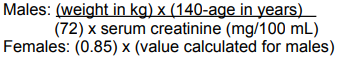 **Patients with Hepatic Insufficiency:** No dose adjustment recommendations can be made in patients with impaired hepatic function. No dosage adjustment is recommended based on age (13 years of age and older) or gender.
INTRAVENOUS, INTRAMUSCULAR
Medical Information
**Therapeutic indications:** Ertapenem is indicated for the treatment of patients with the following moderate to severe infections caused by susceptible isolates of the designated microorganisms. - Complicated Intra-abdominal Infections. - Complicated Skin and Skin Structure Infections including diabetic foot infections without osteomyelitis. - Community-Acquired Pneumonia. - Complicated Urinary Tract Infections including pyelonephritis. - Acute Pelvic Infections including postpartum endomyometritis, septic abortion and post-surgical gynecologic infections. Ertapenem is indicated in adults for the prophylaxis of surgical site infection following elective colorectal surgery. Consideration should be given to official guidance on the appropriate use of antibacterial agents.
**Contraindications:** Ertapenem is contraindicated in patients with known hypersensitivity to any component of this product or to other drugs in the same class or in patients who have demonstrated anaphylactic reactions to beta-lactams. Due to the use of lidocaine HCl as a diluent, Ertapenem administered intramuscularly is contraindicated in patients with a known hypersensitivity to local anesthetics of the amide-type and in patients with severe shock or heart block. (Refer to the prescribing information for lidocaine HCl.).
J01DH03
ertapenem
Manufacturer Information
APOTHECA MARKETING PTE LTD
EUGIA PHARMA SPECIALITIES LIMITED
Active Ingredients
Documents
Package Inserts
Ertapik For Injection 1g(vial) PI.pdf
Approved: October 19, 2021
