Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**4.2. Posology and method of administration** XELJANZ has not been studied and its use should be avoided in combination with tumor necrosis factor (TNF) antagonists, IL-1R antagonists, IL-6R antagonists, anti-CD20 monoclonal antibodies, IL-17 antagonists, IL-12/IL-23 antagonists, anti-integrins, selective co-stimulation modulators and potent immunosuppressants, such as azathioprine, cyclosporine, and tacrolimus because of the possibility of increased immunosuppression and increased risk of infection. XELJANZ treatment should be interrupted if a patient develops a serious infection until the infection is controlled. Posology _**Rheumatoid Arthritis**_ The recommended posology is 5 mg administered twice daily in combination with methotrexate. Monotherapy may be considered in cases of intolerance to methotrexate. _**Psoriatic Arthritis**_ The recommended dose is 5 mg administered twice daily, which should not be exceeded. _**Ulcerative Colitis**_ _Induction treatment_ The recommended dose is 10 mg given orally twice daily for induction for 8 weeks. For patients who do not achieve adequate therapeutic benefit by Week 8, the induction dose of 10 mg twice daily can be extended for an additional 8 weeks (16 weeks total), followed by 5 mg twice daily for maintenance. XELJANZ induction therapy should be discontinued in any patient who shows no evidence of therapeutic benefit by Week 16. _Maintenance treatment_ The recommended dose for maintenance treatment is XELJANZ 5 mg given orally twice daily. XELJANZ 10 mg twice daily for maintenance treatment is not recommended in patients with UC who have known venous thromboembolism (VTE) risk factors, unless there is no suitable alternative treatment available (see Sections 4.4 Special warnings and precautions for use and 4.8 Undesirable effects – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). For patients with UC who are not at increased risk for VTE (see Section 4.4 Special warnings and precautions for use – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_), XELJANZ 10 mg orally twice daily may be considered if the patient experiences a decrease in response on XELJANZ 5 mg twice daily and failed to respond to alternative treatment options for ulcerative colitis such as tumor necrosis factor inhibitor (TNF inhibitor) treatment. XELJANZ 10 mg twice daily for maintenance treatment should be used for the shortest duration possible. The lowest effective dose needed to maintain response should be used. In patients who have responded to treatment with XELJANZ, corticosteroids may be reduced and/or discontinued in accordance with standard of care. _Retreatment in UC_ If therapy is interrupted, restarting treatment with XELJANZ can be considered. If there has been a loss of response, re-induction with XELJANZ 10 mg twice daily may be considered. The treatment interruption period in clinical studies extended up to 1 year. Efficacy may be regained by 8 weeks of 10 mg twice daily therapy (see Section 5.1 Pharmacodynamic properties – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Method of Administration XELJANZ is given orally with or without food. Dose Adjustments Due to Laboratory Abnormalities (see Section 4.4 Special warnings and precautions for use – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) Dose adjustment or interruption of dosing may be needed for management of dose-related laboratory abnormalities including lymphopenia, neutropenia and anemia as described in Tables 1, 2 and 3 below. It is recommended that XELJANZ not be initiated in patients with a lymphocyte count less than 500 cells/mm3.  It is recommended that XELJANZ not be initiated in patients with an absolute neutrophil count (ANC) <1000 cells/mm3. 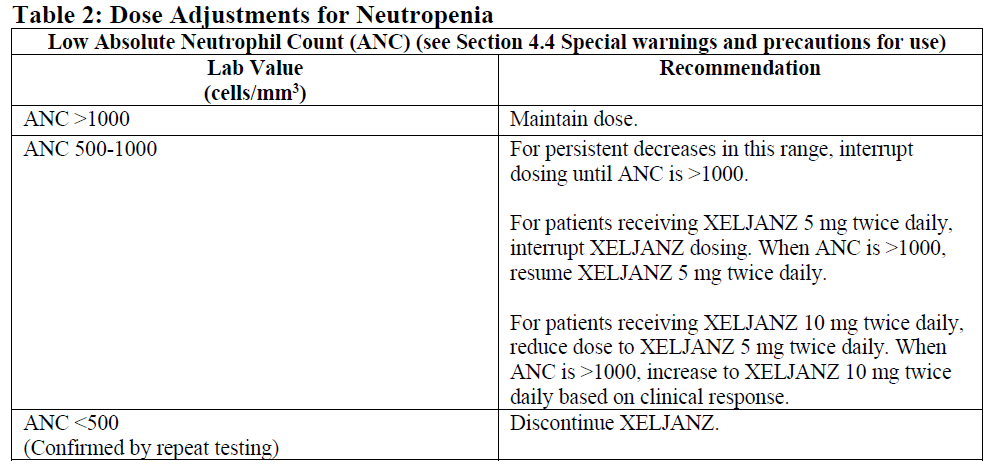 It is recommended that XELJANZ not be initiated in patients with hemoglobin <9 g/dL. 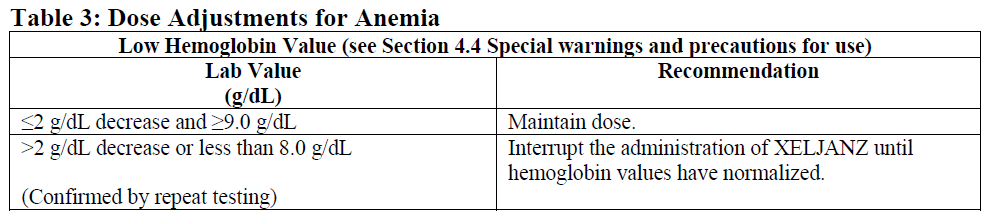 Special Populations _Renal Impairment_ If XELJANZ dose is 5 mg twice daily, the recommended dose in patients with severe renal impairment is XELJANZ 5 mg once daily (see Sections 4.4 Special warnings and precautions for use and 5.2 Pharmacokinetic properties – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Specific recommendations for each indication are provided below. _Rheumatoid Arthritis_ No dose adjustment is required in patients with mild renal impairment. XELJANZ dosage should be reduced to 5 mg once daily in patients with moderate or severe renal impairment (including but not limited to those undergoing hemodialysis) (see Sections 4.4 Special warnings and precautions for use and 5.2 Pharmacokinetic properties – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Psoriatic Arthritis_ No dose adjustment is required in patients with mild renal impairment. XELJANZ dosage should be reduced to 5 mg once daily in patients with moderate or severe renal impairment (including but not limited to those undergoing hemodialysis) (see Section 4.4 Special warnings and precautions for use and 5.2 Pharmacokinetic properties – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Ulcerative Colitis_ No dose adjustment is required in patients with mild or moderate renal impairment. In patients with severe renal impairment (including but not limited to those undergoing hemodialysis), the recommended XELJANZ dose is 5 mg once daily if the dose in the presence of normal renal function is 5 mg twice daily (see Sections 4.4 Special warnings and precautions for use and 5.2 Pharmacokinetic properties – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). In patients with severe renal impairment (including but not limited to those undergoing hemodialysis), the recommended XELJANZ dose is 5 mg twice daily if the dose in the presence of normal renal function is 10 mg twice daily (see Sections 4.4 Special warnings and precautions for use and 5.2 Pharmacokinetic properties – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Hepatic Impairment_ No dose adjustment is required in patients with mild hepatic impairment. If XELJANZ dose is 5 mg twice daily, the recommended dose in patients with moderate hepatic impairment, is XELJANZ 5 mg once daily. _Rheumatoid Arthritis_ No dose adjustment is required in patients with mild hepatic impairment. XELJANZ should not be used in patients with severe hepatic impairment. XELJANZ dosage should be reduced to 5 mg once daily in patients with moderate hepatic impairment (see Sections 4.4 Special warnings and precautions for use and 5.2 Pharmacokinetic properties – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Psoriatic Arthritis_ No dose adjustment is required in patients with mild hepatic impairment. XELJANZ should not be used in patients with severe hepatic impairment. The recommended XELJANZ dose is 5 mg once daily in patients with moderate hepatic impairment (see Sections 4.4 Special warnings and precautions for use and 5.2 Pharmacokinetic properties – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Ulcerative Colitis_ No dose adjustment is required in patients with mild hepatic impairment. XELJANZ should not be used in patients with severe hepatic impairment. In patients with moderate hepatic impairment, the recommended XELJANZ dose is 5 mg twice daily when the indicated dose in the presence of normal hepatic function is 10 mg twice daily, and the recommended dose is 5 mg once daily when the indicated dose in the presence of normal hepatic function is 5 mg twice daily. _Patients Receiving Inhibitors of Cytochrome P450 (CYP3A4) and Cytochrome 2C19 (CYP2C19)_ For indications with a maximum recommended dose of XELJANZ 5 mg twice daily in patients receiving potent inhibitors of CYP3A4 (e.g., ketoconazole) or one or more concomitant medications that result in both moderate inhibition of CYP3A4 and potent inhibition of CYP2C19 (e.g., fluconazole), the recommended dose is XELJANZ 5 mg once daily. Specific recommendations for each indication are provided below. _Rheumatoid Arthritis_ XELJANZ dosage should be reduced to 5 mg once daily in patients receiving potent inhibitors of CYP3A4 (e.g., ketoconazole). XELJANZ dosage should be reduced to 5 mg once daily in patients receiving one or more concomitant medications that result in both moderate inhibition of CYP3A4 and potent inhibition of CYP2C19 (e.g., fluconazole). _Psoriatic Arthritis_ XELJANZ dosage should be reduced to 5 mg once daily in patients receiving potent inhibitors of CYP3A4 (e.g., ketoconazole). XELJANZ dosage should be reduced to 5 mg once daily in patients receiving one or more concomitant medications that result in both moderate inhibition of CYP3A4 and potent inhibition of CYP2C19 (e.g., fluconazole). _Ulcerative Colitis_ In patients receiving potent inhibitors of CYP3A4 (e.g., ketoconazole) or one or more concomitant medications that result in both moderate inhibition of CYP3A4 and potent inhibition of CYP2C19 (e.g., fluconazole), the XELJANZ dose should be reduced to 5 mg twice daily if the patient is taking 10 mg twice daily, and the XELJANZ dose should be reduced to 5 mg once daily if the patient is taking 5 mg twice daily. _Patients Receiving Inducers of Cytochrome P450 (CYP3A4)_ Co-administration of XELJANZ with potent CYP inducers (e.g., rifampin) may result in loss of or reduced clinical response (see Section 4.5 Interaction with other medicinal products and other forms of interaction – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Co-administration of potent inducers of CYP3A4 with XELJANZ is not recommended. _Elderly Patients (≥65 years)_ No dosage adjustment is required in patients aged 65 years and older. _Pediatric_ The safety and efficacy of XELJANZ in children <18 years of age has not yet been established.
ORAL
Medical Information
**4.1. Therapeutic indications** **Rheumatoid Arthritis** XELJANZ (tofacitinib), in combination with methotrexate (MTX), is indicated for reducing the signs and symptoms of rheumatoid arthritis (RA), in adult patients with moderately to severely active RA who have had an inadequate response to MTX. In cases of intolerance to MTX, physicians may consider the use of XELJANZ (tofacitinib) as monotherapy. **Psoriatic Arthritis** XELJANZ (tofacitinib) in combination with MTX is indicated for the treatment of active psoriatic arthritis (PsA) in adult patients who have had an inadequate response or who have been intolerant to a prior disease-modifying antirheumatic drug (DMARD) therapy (see Section 5.1 Pharmacodynamic properties – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Ulcerative Colitis** XELJANZ (tofacitinib) is indicated for the treatment of adult patients with moderately to severely active ulcerative colitis (UC) who have had an inadequate response, lost response, or were intolerant to either conventional therapy or a biologic agent (see Section 5.1 Pharmacodynamic properties – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
**4.3. Contraindications** None.
L04AA29
xl 04 aa 29
Manufacturer Information
PFIZER PRIVATE LIMITED
Pfizer Manufacturing Deutschland GmbH
Active Ingredients
Documents
Package Inserts
Xeljanz_PI.pdf
Approved: March 29, 2023
