Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INFUSION, SOLUTION CONCENTRATE
**2\. DOSAGE AND ADMINISTRATION** **2.1 General** **Patient Selection** _For single-agent treatment of Non-Small Cell Lung Carcinoma, Urothelial Carcinoma, or Colorectal Cancer._ Select patients for treatment with KEYTRUDA based on the presence of positive PD-L1 expression _\[see Clinical Studies (9)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_ in: - locally advanced or metastatic NSCLC. - locally advanced or metastatic urothelial carcinoma who are not eligible for cisplatin-containing chemotherapy. Select patients for treatment with KEYTRUDA based on microsatellite instability-high cancer (MSI-H) or mismatch repair deficient (dMMR) tumor status _\[see Clinical Studies (9)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_ in: - metastatic CRC. _For treatment of TNBC in combination with chemotherapy_ Select patients for treatment with KEYTRUDA based on the presence of positive PD-L1 expression _\[see Clinical Studies (9)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_ in: - locally recurrent unresectable or metastatic TNBC. _For treatment of cervical cancer in combination with chemotherapy, with or without bevacizumab_ Select patients for treatment with KEYTRUDA based on the presence of positive PD-L1 expression _\[see Clinical Studies (9)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_ in: - persistent, recurrent, or metastatic cervical cancer. **Recommended Dosing** KEYTRUDA is administered as an intravenous infusion over 30 minutes. The recommended dose of KEYTRUDA in adults with HNSCC, cHL, urothelial carcinoma, esophageal carcinoma, CRC, HCC, cervical cancer, RCC, endometrial carcinoma, TNBC, previously untreated NSCLC, or for the adjuvant treatment of melanoma, NSCLC or RCC is either: - 200 mg every 3 weeks or - 400 mg every 6 weeks. The recommended dose of KEYTRUDA in adults with previously treated NSCLC or for unresectable or metastatic melanoma is 2 mg/kg every 3 weeks. For use in combination, see the prescribing information for the concomitant therapies. When administering KEYTRUDA as part of a combination with intravenous chemotherapy, KEYTRUDA should be administered first. For RCC patients treated with KEYTRUDA in combination with axitinib, see the prescribing information regarding dosing of axitinib. When used in combination with KEYTRUDA, dose escalation of axitinib above the initial 5 mg dose may be considered at intervals of six weeks or longer _\[see Clinical Studies (9)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. For endometrial carcinoma and RCC patients treated with KEYTRUDA in combination with lenvatinib, the recommended initial dose of lenvatinib is 20 mg orally once daily until disease progression or unacceptable toxicity. Patients should be treated with KEYTRUDA until disease progression or unacceptable toxicity. Atypical responses (i.e., an initial transient increase in tumor size or small new lesions within the first few months followed by tumor shrinkage) have been observed. Clinically stable patients with initial evidence of disease progression should remain on treatment until disease progression is confirmed. For the adjuvant treatment of melanoma, NSCLC or RCC, KEYTRUDA should be administered for up to one year or until disease recurrence or unacceptable toxicity. For the neoadjuvant and adjuvant treatment of high-risk early-stage TNBC, patients should be treated with neoadjuvant KEYTRUDA in combination with chemotherapy for 8 doses of 200 mg every 3 weeks or 4 doses of 400 mg every 6 weeks or until disease progression that precludes definitive surgery or unacceptable toxicity, followed by adjuvant treatment with KEYTRUDA as monotherapy for 9 doses of 200 mg every 3 weeks or 5 doses of 400 mg every 6 weeks or until disease recurrence or unacceptable toxicity. Patients who experience disease progression that precludes definitive surgery or unacceptable toxicity related to KEYTRUDA as neoadjuvant treatment in combination with chemotherapy should not receive KEYTRUDA monotherapy as adjuvant treatment. **Dose Modifications** No dose reductions of KEYTRUDA are recommended. Withhold or discontinue KEYTRUDA to manage adverse reactions as described in Table 1. 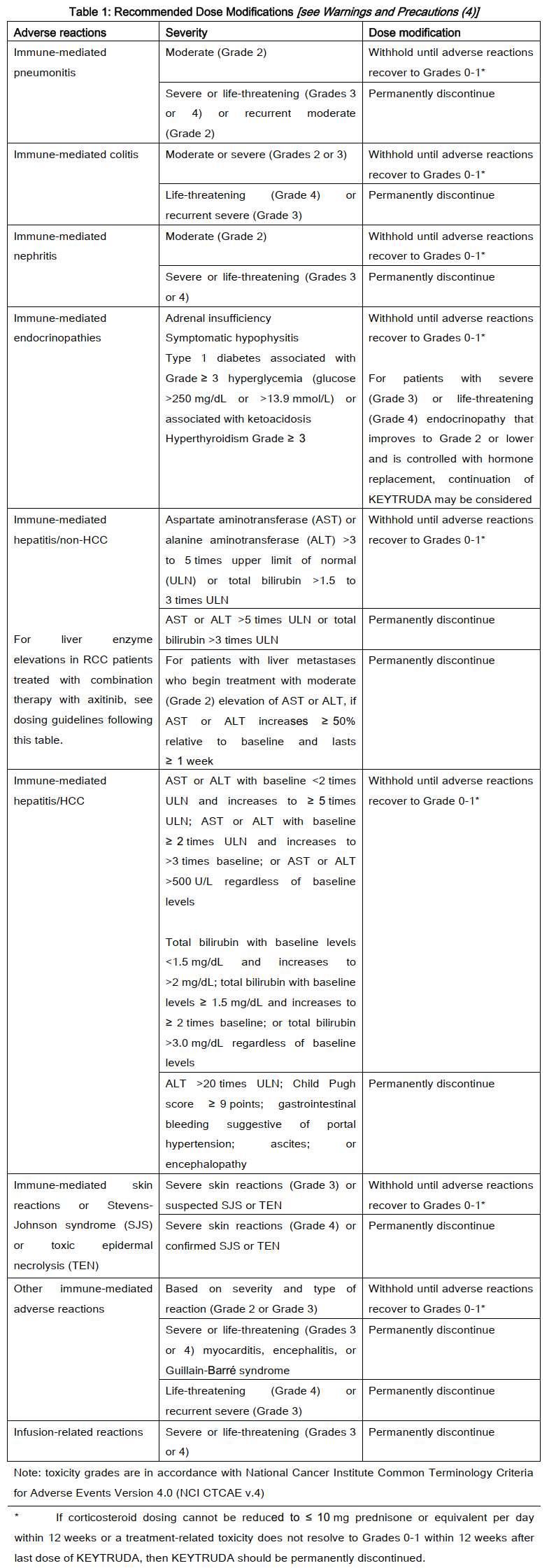 In patients with cHL with Grade 4 hematological toxicity, KEYTRUDA should be withheld until adverse reactions recover to Grades 0–1. In patients with RCC being treated with KEYTRUDA in combination with axitinib: - If ALT or AST ≥ 3 times ULN but <10 times ULN without concurrent total bilirubin ≥ 2 times ULN, withhold both KEYTRUDA and axitinib until these adverse reactions recover to Grades 0–1. Consider corticosteroid therapy. Consider rechallenge with a single drug or sequential rechallenge with both drugs after recovery. If rechallenging with axitinib, consider dose reduction as per the axitinib prescribing information. - If ALT or AST ≥ 10 times ULN or >3 times ULN with concurrent total bilirubin ≥ 2 times ULN, permanently discontinue both KEYTRUDA and axitinib and consider corticosteroid therapy. When administering KEYTRUDA in combination with lenvatinib, interrupt one or both or dose reduce or discontinue lenvatinib to manage adverse reactions as appropriate. For recommendations for management of adverse reactions of lenvatinib, refer to the prescribing information for lenvatinib. No dose reductions are recommended for KEYTRUDA. **Preparation and Administration** - Protect from light. Do not freeze. Do not shake. - Equilibrate the vial of KEYTRUDA to room temperature. - Prior to dilution, the vial of liquid can be out of refrigeration (temperatures at or below 25°C) for up to 24 hours. - Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration. KEYTRUDA is a clear to slightly opalescent, colorless to slightly yellow solution. Discard the vial if visible particles are observed. - Withdraw the required volume up to 4 mL (100 mg) of KEYTRUDA and transfer into an intravenous bag containing 0.9% sodium chloride or 5% glucose (dextrose) to prepare a diluted solution with a final concentration ranging from 1 to 10 mg/mL. Mix diluted solution by gentle inversion. - Do not freeze the infusion solution. - The product does not contain preservative. The diluted product should be used immediately. If not used immediately, diluted solutions of KEYTRUDA may be stored at room temperature (at or below 25°C) for a cumulative time of up to 6 hours. Diluted solutions of KEYTRUDA may also be stored under refrigeration at 2°C to 8°C; however, the total time from dilution of KEYTRUDA to completion of infusion should not exceed 96 hours. If refrigerated, allow the vials and/or IV bags to come to room temperature prior to use. - Translucent to white proteinaceous particles may be seen in the diluted solution. Administer infusion solution intravenously over 30 minutes using a sterile, non-pyrogenic, low-protein binding 0.2 to 5 micrometres in-line or add-on filter. - Do not co-administer other drugs through the same infusion line. - Discard any unused portion left in the vial. **2.2 Pediatric Patients** In melanoma and cHL, the recommended dose of KEYTRUDA in pediatric patients is 2 mg/kg (up to a maximum of 200 mg), administered as an intravenous infusion over 30 minutes every 3 weeks. **2.3 Geriatric Patients** No overall differences in safety or efficacy were reported between elderly patients (65 years and over) and younger patients (less than 65 years). No dose adjustment is necessary in this population. **2.4 Renal Impairment** No dose adjustment is needed for patients with mild or moderate renal impairment. KEYTRUDA has not been studied in patients with severe renal impairment. **2.5 Hepatic Impairment** No dose adjustment is needed for patients with mild or moderate hepatic impairment. KEYTRUDA has not been studied in patients with severe hepatic impairment.
INTRAVENOUS
Medical Information
**1\. INDICATIONS AND USAGE** _Melanoma_ KEYTRUDA (pembrolizumab) is indicated for the treatment of patients with unresectable or metastatic melanoma. KEYTRUDA is indicated for the adjuvant treatment of adult and pediatric (12 years and older) patients with Stage IIB, IIC or III melanoma who have undergone complete resection. _Non-Small Cell Lung Carcinoma_ KEYTRUDA, in combination with pemetrexed and platinum chemotherapy, is indicated for the first-line treatment of patients with metastatic non-squamous non-small cell lung carcinoma (NSCLC), with no EGFR or ALK genomic tumor aberrations. KEYTRUDA, in combination with carboplatin and either paclitaxel or nab-paclitaxel, is indicated for the first-line treatment of patients with metastatic squamous NSCLC. KEYTRUDA as monotherapy is indicated for the first-line treatment of patients with metastatic NSCLC whose tumors express PD-L1 with a ≥ 50% tumor proportion score (TPS) as determined by a validated test, with no EGFR or ALK genomic tumor aberrations. KEYTRUDA as monotherapy is indicated for the treatment of patients with locally advanced or metastatic NSCLC whose tumors express PD-L1 with a ≥ 1% TPS as determined by a validated test and who have received platinum-containing chemotherapy. Patients with EGFR or ALK genomic tumor aberrations should have received prior therapy for these aberrations prior to receiving KEYTRUDA. KEYTRUDA, as monotherapy, is indicated as adjuvant treatment following resection and platinum-based chemotherapy for adults patients with Stage IB (T2a ≥ 4 cm), II, or IIIA NSCLC. _Head and Neck Cancer_ KEYTRUDA, as monotherapy or in combination with platinum and 5-fluorouracil (5-FU) chemotherapy, is indicated for the first line treatment of patients with metastatic or unresectable recurrent head and neck squamous cell carcinoma (HNSCC) whose tumours express PD-L1 with a CPS ≥ 1. _Classical Hodgkin Lymphoma_ KEYTRUDA is indicated for the treatment of adult and pediatric patients aged 3 years and above, with relapsed or refractory classical Hodgkin lymphoma (cHL), who have failed autologous stem cell transplant (ASCT) or following at least two prior therapies when ASCT is not a treatment option. _Urothelial Carcinoma_ KEYTRUDA is indicated for the treatment of patients with locally advanced or metastatic urothelial carcinoma whose tumours express PD-L1 with a combined positive score (CPS) ≥ 10 as determined by a validated test, and who are not eligible for cisplatin-containing chemotherapy. KEYTRUDA is indicated for the treatment of patients with locally advanced or metastatic urothelial carcinoma who have received prior platinum-containing chemotherapy. _Esophageal Cancer_ KEYTRUDA, in combination with platinum and fluoropyrimidine based chemotherapy, is indicated for the first-line treatment of patients with locally advanced or metastatic carcinoma of the esophagus or HER2 negative gastroesophageal junction (GEJ) adenocarcinoma (tumors with epicenter 1 to 5 centimeters above the GEJ) that is not amenable to surgical resection or definitive chemoradiation. _Colorectal Cancer_ KEYTRUDA is indicated for the first-line treatment of patients with metastatic microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) colorectal cancer (CRC). _Hepatocellular Carcinoma_ KEYTRUDA is indicated for the treatment of patients with hepatocellular carcinoma (HCC) who have been previously treated with an anti-angiogenic tyrosine kinase inhibitor (TKI). _Cervical Cancer_ KEYTRUDA, in combination with chemotherapy with or without bevacizumab, is indicated for the treatment of patients with persistent, recurrent, or metastatic cervical cancer whose tumours express PD-L1 with a CPS ≥ 1 _\[see Clinical Studies (9)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. _Renal Cell Carcinoma_ KEYTRUDA, in combination with axitinib, is indicated for the first-line treatment of patients with advanced renal cell carcinoma (RCC). KEYTRUDA, in combination with lenvatinib, is indicated for the first-line treatment of patients with advanced RCC. KEYTRUDA, as monotherapy, is indicated for the adjuvant treatment of patients with RCC at increased risk of recurrence following nephrectomy, or following nephrectomy and resection of metastatic lesions _\[see Clinical Studies (9)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. _Endometrial Carcinoma_ KEYTRUDA, in combination with lenvatinib, is indicated for the treatment of patients with advanced endometrial carcinoma who have disease progression following prior systemic therapy in any setting and are not candidates for curative surgery or radiation. _Triple-Negative Breast Cancer_ KEYTRUDA is indicated for the treatment of patients with high-risk early-stage triple-negative breast cancer (TNBC) in combination with chemotherapy as neoadjuvant treatment, and then continued as monotherapy as adjuvant treatment after surgery. KEYTRUDA, in combination with chemotherapy, is indicated for the treatment of patients with locally recurrent unresectable or metastatic TNBC whose tumors express PD-L1 (CPS ≥ 10) as determined by a validated test and who have not received prior chemotherapy for metastatic disease _\[see Clinical Studies (9)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_.
**3\. CONTRAINDICATIONS** KEYTRUDA is contraindicated in patients with hypersensitivity to pembrolizumab or any of the inactive ingredients.
L01FF02
pembrolizumab
Manufacturer Information
MSD PHARMA (SINGAPORE) PTE. LTD.
MSD International GmbH T/A MSD Ireland (Carlow)
Active Ingredients
Documents
Package Inserts
Keytruda PI.pdf
Approved: May 17, 2023
