Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**4.2 Posology and method of administration** Posology _Prevention of stroke and systemic embolism in patients with non-valvular atrial fibrillation (NVAF)_ The recommended dose of apixaban is 5 mg taken orally twice daily. _Dose reduction_ The recommended dose of apixaban is 2.5 mg taken orally twice daily in patients with NVAF and at least two of the following characteristics: age ≥80 years, body weight ≤60 kg, or serum creatinine ≥1.5 mg/dL (133 micromole/L). Therapy should be continued long term. _Treatment of DVT, treatment of PE and prevention of recurrent DVT and PE (VTEt)_ The recommended dose of apixaban for the treatment of acute DVT and treatment of PE is 10 mg taken orally twice daily for the first 7 days followed by 5 mg taken orally twice daily. As per available medical guidelines, short duration of treatment (at least 3 months) should be based on transient risk factors (e.g., recent surgery, trauma, immobilisation). The recommended dose of apixaban for the prevention of recurrent DVT and PE is 2.5 mg taken orally twice daily. When prevention of recurrent DVT and PE is indicated, the 2.5 mg twice daily dose should be initiated following completion of 6 months of treatment with apixaban 5 mg twice daily or with another anticoagulant, as indicated in Table 1 below (see also section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). 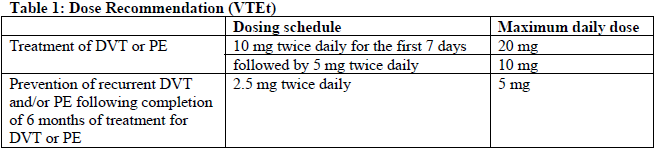 The duration of overall therapy should be individualised after careful assessment of the treatment benefit against the risk for bleeding (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Missed dose_ If a dose is missed, the patient should take ELIQUIS immediately and then continue with twice daily intake as before. _Switching_ Switching treatment from parenteral anticoagulants to ELIQUIS (and _vice versa_) can be done at the next scheduled dose (see section 4.5 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). These medicinal products should not be administered simultaneously. _Switching from vitamin K antagonist (VKA) therapy to ELIQUIS_ When converting patients from vitamin K antagonist (VKA) therapy to ELIQUIS, warfarin or other VKA therapy should be discontinued and ELIQUIS started when the international normalised ratio (INR) is <2. _Switching from ELIQUIS to VKA therapy_ When converting patients from ELIQUIS to VKA therapy, administration of ELIQUIS should be continued for at least 2 days after beginning VKA therapy. After 2 days of co-administration of ELIQUIS with VKA therapy, an INR should be obtained prior to the next scheduled dose of ELIQUIS. Co-administration of ELIQUIS and VKA therapy should be continued until the INR is ≥2. _Elderly_ VTEt – No dose adjustment required (see sections 4.4 and 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). NVAF – No dose adjustment required, unless criteria for dose reduction are met (see _Dose reduction_ at the beginning of section 4.2). _Renal impairment_ In patients with mild or moderate renal impairment, the following recommendations apply: - for the treatment of DVT, treatment of PE and prevention of recurrent DVT and PE (VTEt), no dose adjustment is necessary (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). - for the prevention of stroke and systemic embolism in patients with NVAF and serum creatinine ≥1.5 mg/dL (133 micromole/L) associated with age ≥80 years or body weight ≤60 kg, a dose reduction is necessary and described above. In the absence of other criteria for dose reduction (age, body weight), no dose adjustment is necessary (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). In patients with severe renal impairment (creatinine clearance 15–29 mL/min) the following recommendations apply (see sections 4.4 and 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_): - for the treatment of DVT, treatment of PE and prevention of recurrent DVT and PE (VTEt) apixaban is to be used with caution; - for the prevention of stroke and systemic embolism in patients with NVAF, patients should receive the lower dose of apixaban 2.5 mg twice daily. In patients with creatinine clearance <15 mL/min, or in patients undergoing dialysis, there is no clinical experience therefore apixaban is not recommended in these patients (see sections 4.4 and 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Hepatic impairment_ ELIQUIS is contraindicated in patients with hepatic disease associated with coagulopathy and clinically relevant bleeding risk (see section 4.3). It is not recommended in patients with severe hepatic impairment (see sections 4.4 and 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). It should be used with caution in patients with mild or moderate hepatic impairment (Child-Pugh A or B). No dose adjustment is required in patients with mild or moderate hepatic impairment (see sections 4.4 and 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Patients with elevated liver enzymes alanine aminotransferase (ALT)/aspartate aminotransferase (AST) >2 x ULN or total bilirubin ≥1.5 x ULN were excluded in clinical studies. Therefore, ELIQUIS should be used with caution in this population (see sections 4.4 and 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Prior to initiating ELIQUIS, liver function testing should be performed. _Body weight_ VTEt – No dose adjustment required (see sections 4.4 and 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). NVAF – No dose adjustment required, unless criteria for dose reduction are met (see _Dose reduction_ at the beginning of section 4.2). _Gender_ No dose adjustment required (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Patients undergoing catheter ablation (NVAF)_ Patients can continue apixaban use while undergoing catheter ablation (see sections 4.3, 4.4 and 4.5 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _NVAF patients undergoing cardioversion_ Patients can stay on apixaban while being cardioverted. Confirmation should be sought prior to cardioversion that the patient has taken apixaban as prescribed. _Patients with NVAF and acute coronary syndrome (ACS) and/or percutaneous coronary intervention (PCI)_ There is limited experience of treatment with apixaban at the recommended dose for NVAF patients when used in combination with antiplatelet agents in patients with ACS and/or undergoing PCI after haemostasis is achieved (see sections 4.4 and 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Paediatric population_ The safety and efficacy of ELIQUIS in children and adolescents below age 18 have not been established. Currently available data on thromboembolism prevention are described in section 5.1 but no recommendation on a posology can be made – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. Method of administration Oral use. ELIQUIS should be swallowed with water, with or without food. For patients who are unable to swallow whole tablets, ELIQUIS tablets may be crushed and suspended in water, or 5% glucose in water (G5W), or apple juice or mixed with apple puree and immediately administered orally (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Alternatively, ELIQUIS tablets may be crushed and suspended in 60 mL of water or G5W and immediately delivered through a nasogastric tube (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Crushed ELIQUIS tablets are stable in water, G5W, apple juice, and apple puree for up to 4 hours.
ORAL
Medical Information
**4.1 Therapeutic indications** Prevention of stroke and systemic embolism in adult patients with non-valvular atrial fibrillation (NVAF), with one or more risk factors, such as prior stroke or transient ischaemic attack (TIA); age ≥75 years; hypertension; diabetes mellitus; symptomatic heart failure (NYHA Class ≥II). Treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE), and prevention of recurrent DVT and PE in adults (see section 4.4 for haemodynamically unstable PE patients – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
**4.3 Contraindications** - Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. - Active clinically significant bleeding. - Hepatic disease associated with coagulopathy and clinically relevant bleeding risk (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). - Lesion or condition if considered a significant risk factor for major bleeding. This may include current or recent gastrointestinal ulceration, presence of malignant neoplasms at high risk of bleeding, recent brain or spinal injury, recent brain, spinal or ophthalmic surgery, recent intracranial haemorrhage, known or suspected oesophageal varices, arteriovenous malformations, vascular aneurysms or major intraspinal or intracerebral vascular abnormalities. - Concomitant treatment with any other anticoagulant agent e.g., unfractionated heparin (UFH), low molecular weight heparins (enoxaparin, dalteparin, etc.), heparin derivatives (fondaparinux, etc.), oral anticoagulants (warfarin, rivaroxaban, dabigatran, etc.) except under specific circumstances of switching anticoagulant therapy (see section 4.2), when UFH is given at doses necessary to maintain an open central venous or arterial catheter or when UFH is given during catheter ablation for atrial fibrillation (see sections 4.4 and 4.5 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
B01AF02
apixaban
Manufacturer Information
PFIZER PRIVATE LIMITED
Bristol-Myers Squibb Manufacturing Company Unlimited Company
Catalent Anagni S.r.l. (Primary and secondary packager)
Pfizer Ireland Pharmaceuticals
Pfizer Manufacturing Deutschland GmbH (Primary and secondary packager)
Active Ingredients
Documents
Package Inserts
Eliquis Tablet 5mg PI and PIL.pdf
Approved: January 25, 2023
