Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**Dosage and Administration** Pharmaceutical form: Film-coated tablets Therapy should be initiated by a physician experienced in the management of HIV infection. Before taking maraviroc it has to be confirmed that only CCR5-tropic HIV-1 is detectable (i.e. CXCR4 or dual/mixed tropic virus not detected) using an adequately validated and sensitive detection method on a newly drawn blood sample. The Monogram Trofile assay was used in the clinical studies of maraviroc. Other phenotypic and genotypic assays are currently being evaluated. The viral tropism cannot be safely predicted by treatment history and assessment of stored samples. There are currently no data regarding the reuse of maraviroc in patients that currently have only CCR5-tropic HIV-1 detectable, but have a history of failure on maraviroc (or other CCR5 antagonists) with a CXCR4 or dual/mixed tropic virus. There are no data regarding the switch from a medicinal product of a different antiretroviral class to maraviroc in virologically suppressed patients. Alternative treatment options should be considered. Adults: the recommended dose of maraviroc is 150 mg, 300 mg or 600 mg twice daily depending on interactions with concomitant antiretroviral therapy and other medicinal products (see _Table 1 and Interactions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Maraviroc can be taken with or without food. 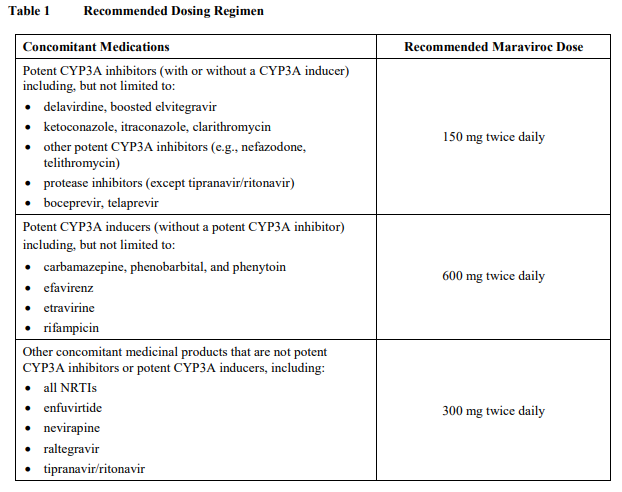 Children: the safety and efficacy for the use of maraviroc in children younger than 18 years of age have not been established, therefore use in children is not recommended ( _see Pharmacokinetics_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Elderly: there is limited experience in patients above 65 years of age. Therefore caution should be exercised when administering maraviroc in elderly patients ( _see Pharmacokinetics_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Renal impairment: dosage adjustment is only recommended in patients with renal impairment who are receiving potent CYP3A inhibitors such as: - protease inhibitors (except tipranavir/ritonavir and fosamprenavir/ritonavir) ( _see Table 2_). - boceprevir, telaprevir - delavirdine, boosted elvitegravir - ketoconazole, itraconazole, clarithromycin, nefazodone, telithromycin. Maraviroc should be used with caution in patients with severe renal impairment (creatinine clearance < 30 mL/min) who are receiving potent CYP3A inhibitors ( _see Warnings and Precautions and Pharmacokinetics_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Maraviroc should be dosed every 24 hours in renally impaired patients (creatinine clearance < 80 mL/min), including patients with end stage renal disease (ESRD) requiring dialysis, who are receiving maraviroc in combination with potent CYP3A inhibitors ( _see Warnings and Precautions, Interactions and Pharmacokinetics_). These dosing recommendations are based on data from a renal impairment study ( _see Pharmacokinetics_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) in addition to modelling of pharmacokinetic data in subjects with varying degrees of renal impairment. No dose adjustment is necessary for renally impaired patients, including patients with ESRD, requiring dialysis, not receiving a potent CYP3A inhibitor in combination with maraviroc. Table 2 below provides dosing interval adjustment guidelines. 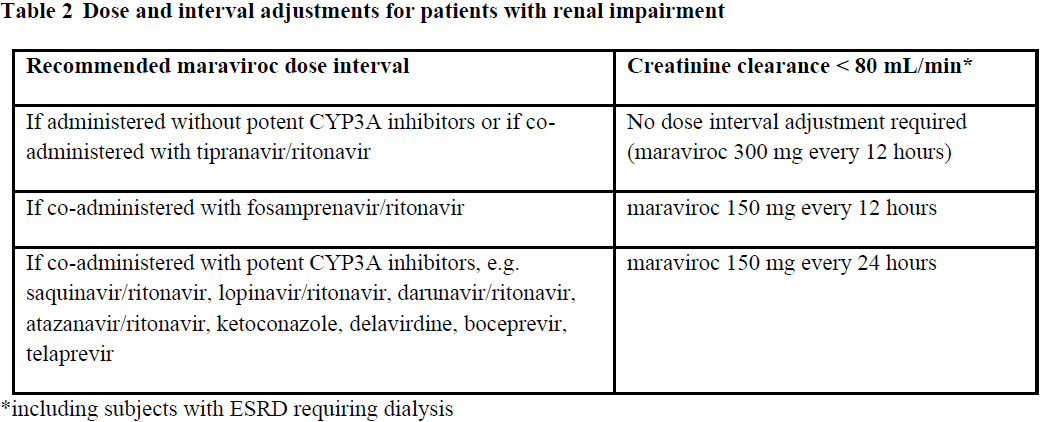 Hepatic impairment: limited data in patients with mild and moderate hepatic impairment demonstrated a small increase in the mean Cmax of maraviroc, suggesting no dose adjustment is required. However, maraviroc should be used with caution in patients with hepatic impairment ( _see Warnings and Precautions and Pharmacokinetics_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
ORAL
Medical Information
**Indications** Maraviroc, in combination with other antiretroviral medicinal products, is indicated for treatment-experienced adult patients infected with only CCR5-tropic HIV-1, who have evidence of viral replication and HIV-1 strains resistant to multiple antiretroviral agents. This indication is based on safety and efficacy data from two double-blind, placebo-controlled trials in treatment-experienced patients ( _see Clinical Studies_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The following points should be considered when initiating therapy with maraviroc: - Treatment history should guide the use of CELSENTRI. Tropism testing is required for the appropriate use of maraviroc ( _see Warnings and Precautions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). - Use of maraviroc is not recommended in patients with dual/mixed or CXCR4-tropic HIV-1 as efficacy was not demonstrated in a phase 2 study of this patient group. - The safety and efficacy of maraviroc have not been established in treatment-naïve adult patients or pediatric patients.
**Contraindications** Hypersensitivity to the active substance or to any of the excipients ( _see Excipients_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
J05AX09
maraviroc
Manufacturer Information
GLAXOSMITHKLINE PTE LTD
Pfizer Manufacturing Deutschland GmbH
Active Ingredients
Documents
Package Inserts
Celsentri Tablet PI.pdf
Approved: February 15, 2023
