Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, DELAYED RELEASE
**2 DOSAGE AND ADMINISTRATION** **2.1 Important Administration Instructions for Posaconazole Delayed-Release Tablets** Posaconazole delayed-release tablets and oral suspension are not to be used interchangeably due to the differences in the dosing of each formulation _\[see Dosage and Administration (2.3), (2.5)\]_. - Swallow tablets whole. Do not divide, crush, or chew. - Patients who have severe diarrhea or vomiting should be monitored closely for breakthrough fungal infections when receiving posaconazole delayed-release tablets. **2.3 Dosage and Administration Instructions for Posaconazole Delayed-Release Tablets** **Dosage:** 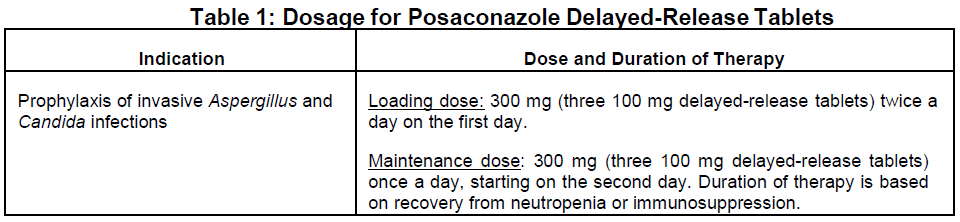 **Administration Instructions for Posaconazole Delayed-Release Tablets:** - Swallow tablets whole. Do not divide, crush, or chew. - Posaconazole delayed-release tablets can be taken without regard to food _\[see Clinical Pharmacology (12)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. - Posaconazole delayed-release tablets should be used only for the prophylaxis indication. - Posaconazole delayed-release tablets generally provide higher plasma drug exposures than posaconazole oral suspension under both fed and fasted conditions, and therefore is the preferred oral formulation for the prophylaxis indication. - Duration of therapy is based on recovery from neutropenia or immunosuppression. For patients with acute myelogenous leukemia or myelodysplastic syndromes, prophylaxis with Posaconazole should start several days before the anticipated onset of neutropenia and continue for 7 days after the neutrophil count rises above 500 cells per mm3. - Dose adjustments in hepatic impairment: There are limited pharmacokinetic data in patients with hepatic insufficiency; therefore, no recommendation for dose adjustment can be made. In the small number of subjects studied who had hepatic insufficiency, there was an increase in half-life with a decrease in hepatic function. - Dose adjustments in children: Safety and efficacy in children below the age of 13 years have not been established. **2.5 Non-Interchangeability between posaconazole delayed-release tablets and posaconazole oral suspension** Posaconazole delayed-release tablets and oral suspension are not to be used interchangeably due to the differences in the dosing of each formulation. Therefore, follow the specific dosage recommendations for each of the formulations _\[see Dosage and Administration (2.3)\]_. **2.6 Dosage Adjustments in Patients with Renal Impairment** The pharmacokinetics of posaconazole delayed-release tablets is not significantly affected by renal impairment. Therefore, no adjustment is necessary for oral dosing in patients with mild to severe renal impairment.
ORAL
Medical Information
**1 INDICATIONS AND USAGE** **1.1 Prophylaxis of Invasive _Aspergillus_ and _Candida_ Infections** Posaconazole delayed-release tablets are indicated for prophylaxis of invasive _Aspergillus_ and _Candida_ infections in patients who are at high risk of developing these infections due to being severely immunocompromised, such as hematopoietic stem cell transplant (HSCT) recipients with graft-versus-host disease (GVHD) or those with hematologic malignancies with prolonged neutropenia from chemotherapy. Posaconazole delayed-release tablets 100 mg are indicated in patients 13 years of age and older.
**4 CONTRAINDICATIONS** POSACONAZOLE is contraindicated in patients with known hypersensitivity to POSACONAZOLE or any component of the product. Although not studied in vitro or in vivo, coadministration of the CYP3A4 substrates terfenadine, astemizole, cisapride, pimozide, or quinidine with POSACONAZOLE are contraindicated since increased plasma concentrations of these drugs can lead to QT prolongation and rare occurrences of torsade de pointes. Coadministration with the HMG-CoA reductase inhibitors that are primarily metabolized through CYP3A4 is contraindicated since increased plasma concentration of these drugs can lead to rhabdomyolysis. Although not studied in vitro or in vivo, POSACONAZOLE may increase the plasma concentrations of ergot alkaloids which may lead to ergotism. Coadministration of POSACONAZOLE and ergot alkaloids is contraindicated.
J02AC04
posaconazole
Manufacturer Information
KINGTOP INTERNATIONAL TRADING PTE. LTD.
HAIMEN PHARMA INC
Active Ingredients
Documents
Package Inserts
Sinotrx Posaconazole Delayed Release Tablet 100mg PI.pdf
Approved: September 19, 2022
