Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**4.2 Posology and method of administration** Patients whose therapy is started with linezolid injection may be switched to linezolid tablets or linezolid for oral suspension, with no dosage adjustment. 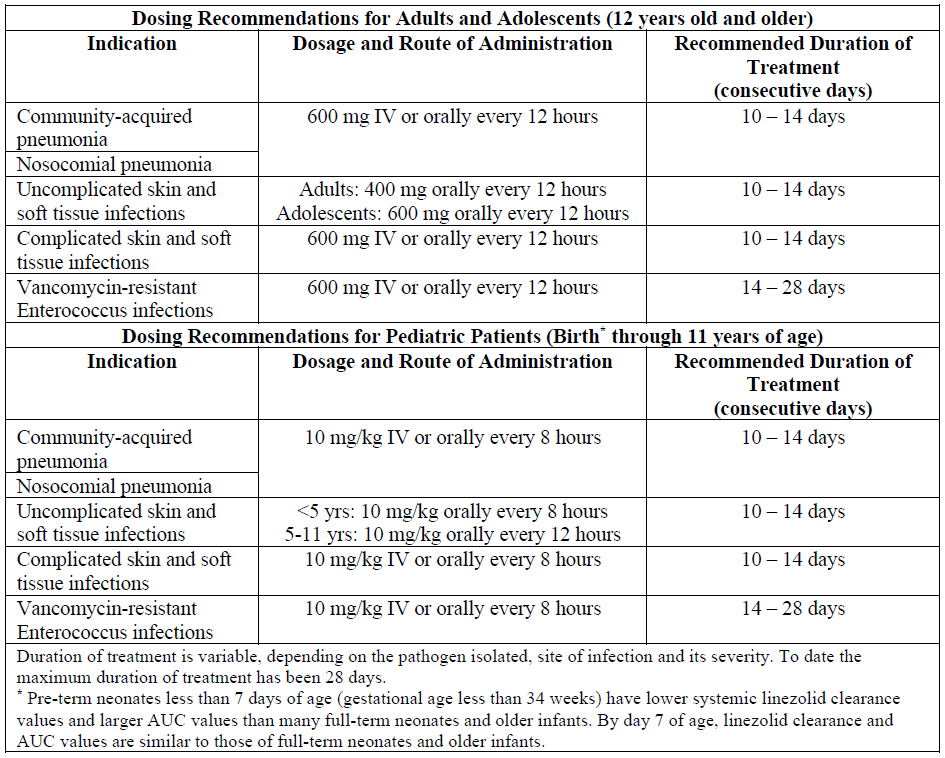 _**Elderly patients:**_ No dose adjustment is required. _**Patients with renal insufficiency:**_ No dose adjustment is required (see section 5.2 Pharmacokinetic properties – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Patients with severe renal insufficiency (i.e., CLCR <30 ml/min): No dose adjustment is required. Due to the unknown clinical significance of higher exposure (up to 10-fold) to the two primary metabolites of linezolid in patients with severe renal insufficiency, linezolid should be used with special caution in these patients and only when the anticipated benefit is considered to outweigh the theoretical risk. As approximately 30% of a linezolid dose is removed during 3 hours of hemodialysis, linezolid should be given after dialysis in patients receiving such treatment. The primary metabolites of linezolid are removed to some extent by hemodialysis, but the concentrations of these metabolites are still very considerably higher following dialysis than those observed in patients with normal renal function or mild to moderate renal insufficiency. Therefore, linezolid should be used with special caution in patients with severe renal insufficiency who are undergoing dialysis and only when the anticipated benefit is considered to outweigh the theoretical risk. To date, there is no experience of linezolid administration to patients undergoing continuous ambulatory peritoneal dialysis (CAPD) or alternative treatments for renal failure (other than hemodialysis). _**Patients with hepatic insufficiency:**_ No dose adjustment is required. However, there are limited clinical data and it is recommended that linezolid should be used in such patients only when the anticipated benefit is considered to outweigh the theoretical risk (see section 5.2 Pharmacokinetic properties – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _**Pediatric:**_ Recommended dosages for pediatric patients please refer to the table above. **Linezolid Injection** Administer linezolid injection by intravenous infusion over a period of 30 to 120 minutes. **Do not use the intravenous infusion bag in series connections**. Do not introduce additives into the intravenous solution. If linezolid injection is to be given concomitantly with another drug, each drug should be given separately, in accordance with the recommended dosage and route of administration for each product. Linezolid injection was physically incompatible with the following drugs when combined in simulated Y-site administration: amphotericin B, chlorpromazine HCl, diazepam, pentamidine isethionate, phenytoin sodium, erythromycin lactobionate and trimethoprim-sulfamethoxazole. Linezolid injection was chemically incompatible when combined with ceftriaxone sodium. Compatible Infusion Solutions: 5% Dextrose Injection 0.9% Sodium Chloride Injection Lactated Ringer’s Injection
ORAL
Medical Information
**4.1 Therapeutic indications** Linezolid is indicated for the treatment of **adults and adolescents (12 years and older) and pediatric patients (birth through 11 years of age)** with the following infections caused by susceptible strains of Gram-positive bacteria only. Linezolid is not indicated for the treatment of Gram-negative infections. Specific Gram-negative therapy is required if a concomitant Gram-negative pathogen is documented or suspected (see sections 4.4 Special warnings and precautions for use and 5.1 Pharmacodynamic properties – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Community-acquired pneumonia caused by _Streptococcus pneumoniae_ (penicillin-sensitive strains only), including cases with concurrent bacteremia, or _Staphylococcus aureus_ (methicillin-sensitive strains only). Nosocomial pneumonia caused by _Staphylococcus aureus_ (methicillin-sensitive and methicillin-resistant strains) or _Streptococcus pneumoniae_ (penicillin-sensitive strains only). Uncomplicated skin and soft tissue infections caused by _Staphylococcus aureus_ (methicillin-sensitive strains only) or _Streptococcus pyogenes_. Complicated skin and soft tissue infections, caused by _Staphylococcus aureus_ (methicillin-sensitive and methicillin-resistant strains), _Streptococcus pyogenes_ or _Streptococcus agalactiae_. Vancomycin-resistant _Enterococcus faecium_ infections including cases with concurrent bacteremia. Linezolid should not be initiated as a first line therapy for community-acquired pneumonia or uncomplicated skin infection, but may be considered if resistant strains are suspected or proven or in presence of drug allergy.
**4.3 Contraindications** Linezolid is contraindicated in patients who have previously demonstrated hypersensitivity to linezolid or any of the other product components. **Monoamine Oxidase Inhibitors** Linezolid should not be used in patients taking any medicinal product which inhibits monoamine oxidases A or B (e.g., phenelzine, isocarboxazid) or within two weeks of taking any such medicinal product. **Potential Interactions Producing Elevation of Blood Pressure** Unless patients are monitored for potential increases in blood pressure, linezolid should not be administered to patients with uncontrolled hypertension, pheochromocytoma, thyrotoxicosis and/or patients taking any of the following types of medications: directly and indirectly acting sympathomimetic agents (e.g., pseudoephedrine, phenylpropanolamine), vasopressive agents (e.g., epinephrine, norepinephrine), dopaminergic agents (e.g., dopamine, dobutamine) (see section 4.5 Interaction with other medicinal products and other forms of interaction – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Potential Serotonergic Interactions** Unless patients are carefully observed for signs and/or symptoms of serotonin syndrome, linezolid should not be administered to patients with carcinoid syndrome and/or patients taking any of the following medications: serotonin re-uptake inhibitors, tricyclic antidepressants, serotonin 5-HT1 receptor agonists (triptans), meperidine or buspirone (see section 4.5 Interaction with other medicinal products and other forms of interaction – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
J01XX08
linezolid
Manufacturer Information
PFIZER PRIVATE LIMITED
Pharmacia & Upjohn Co (Primary packager)
Pfizer Pharmaceuticals LLC (Vega Baja)
Pfizer Manufacturing Deutschland GmbH (Primary & Secondary Packager)
Active Ingredients
Documents
Package Inserts
Zyvox PI.pdf
Approved: May 11, 2022
