Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, SUSPENSION, EXTENDED RELEASE
**Dosage and Administration** For patients who have never taken oral paliperidone or oral or injectable risperidone, it is recommended to establish tolerability with oral paliperidone or oral risperidone prior to initiating treatment with INVEGA® Sustenna®. **Dosage** _Schizophrenia:_ Recommended initiation of INVEGA® Sustenna® is with a dose of 150 mg on treatment day 1 and 100 mg one week later, both administered in the deltoid muscle. The recommended monthly maintenance dose is 75 mg; some patients may benefit from lower or higher doses within the recommended range of 25 to 150 mg based on individual patient tolerability and/or efficacy. Following the second initiation dose, monthly maintenance doses can be administered in either the deltoid or gluteal muscle. _Schizoaffective disorder:_ Recommended initiation of INVEGA® Sustenna® is with a dose of 150 mg on treatment day 1 and 100 mg one week later, both administered in the deltoid muscle. The recommended monthly maintenance dose is within the range of 50 to 150 mg adjusted based on tolerability and/or efficacy using available strengths. The 25 mg strength was not studied in schizoaffective disorder. Following the second initiation dose, monthly maintenance doses can be administered in either the deltoid or gluteal muscle. Adjustment of the maintenance dose may be made monthly. When making dose adjustments, the prolonged-release characteristics of INVEGA® Sustenna® should be considered (see _Pharmacokinetic Properties_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_), as the full effect of the dose adjustment may not be evident for several months. **Missed Doses** _**Avoiding Missed Doses**_ It is recommended that the second initiation dose of INVEGA® Sustenna® be given one week after the first dose. To avoid a missed dose, patients may be given the second dose 2 days before or after the one-week timepoint. Similarly, the third and subsequent injections after the initiation regimen are recommended to be given monthly. To avoid a missed monthly dose, patients may be given the injection up to 7 days before or after the monthly timepoint. If the target date for the second INVEGA® Sustenna® Injection (one week ± 2 days) is missed, the recommended reinitiation depends on the length of time which has elapsed since the patient's first injection. _**Missed second initiation dose (< 4 weeks from first injection)**_ If less than 4 weeks have elapsed since the first injection, then the patient should be administered the second injection of 100 mg in the deltoid muscle as soon as possible. A third INVEGA® Sustenna® injection of 75 mg in either the deltoid or gluteal muscles should be administered 5 weeks after the first injection (regardless of the timing of the second injection). The normal monthly cycle of injections in either the deltoid or gluteal muscle of 25 mg to 150 mg based on individual patient tolerability and/or efficacy should be followed thereafter. _**Missed second initiation dose (4–7 weeks from first injection)**_ If 4 to 7 weeks have elapsed since the first injection of INVEGA® Sustenna®, resume dosing with two injections of 100 mg in the following manner: a deltoid injection as soon as possible followed by another deltoid injection one week later, then resumption of the normal monthly cycle of injections in either the deltoid or gluteal muscle of 25 mg to 150 mg based on individual patient tolerability and/or efficacy. _**Missed second initiation dose (> 7 weeks from first injection)**_ If more than 7 weeks have elapsed since the first injection of INVEGA® Sustenna®, initiate dosing as described for the initial recommended initiation of INVEGA® Sustenna® above. _**Missed Maintenance Dose (1 Month to 6 Weeks)**_ After initiation, the recommended injection cycle of INVEGA® Sustenna® is monthly. If less than 6 weeks have elapsed since the last injection, then the previously stabilized dose should be administered as soon as possible, followed by injections at monthly intervals. _**Missed Maintenance Dose (> 6 Weeks to 6 Months)**_ If more than 6 weeks have elapsed since the last injection of INVEGA® Sustenna®, the recommendation is as follows: _**For patients stabilized with doses of 25 to 100 mg:**_ 1. A deltoid injection as soon as possible at the same dose the patient was previously stabilised on. 2. Another deltoid injection (same dose) one week later (day 8). 3. Resumption of the normal monthly cycle of injections in either the deltoid or gluteal muscle of 25 mg to 150 mg based on individual patient tolerability and/or efficacy. _**For patients stabilized with 150 mg:**_ 1. A deltoid injection as soon as possible at the 100 mg dose. 2. Another deltoid injection one week later (day 8) at the 100 mg dose. 3. Resumption of the normal monthly cycle of injections in either the deltoid or gluteal muscle of 25 mg to 150 mg based on individual patient tolerability and/or efficacy. _**Missed Maintenance Dose (> 6 Months)**_ If more than 6 months have elapsed since the last injection of INVEGA® Sustenna®, initiate dosing as described for the initial recommended initiation of INVEGA® Sustenna® above. **Administration** INVEGA® Sustenna® is intended for intramuscular use only. Inject slowly, deep into the muscle. Care should be taken to avoid inadvertent injection into a blood vessel. Each injection should be administered by a health care professional. Administration should be in a single injection. Do not administer the dose in divided injections. Do not administer intravascularly or subcutaneously. The recommended needle size for administration of INVEGA® Sustenna® into the deltoid muscle is determined by the patient's weight. For those ≥ 90 kg (≥ 200 lb), the 1½ inch, 22-gauge needle is recommended. For those < 90 kg (< 200 lb), the 1-inch, 23-gauge needle is recommended. Deltoid injections should be alternated between the two deltoid muscles. The recommended needle size for administration of INVEGA® Sustenna® into the gluteal muscle is the 1½-inch, 22-gauge needle. Administration should be made into the upper-outer quadrant of the gluteal area. Gluteal injections should be alternated between the two gluteal muscles. Since paliperidone is the major active metabolite of risperidone, caution should be exercised when INVEGA® Sustenna® is coadministered with risperidone or with oral paliperidone for extended periods of time. Safety data involving concomitant use of INVEGA® Sustenna® with other antipsychotics is limited. **Special Populations** _**Pediatrics (less than 18 years of age)**_ Safety and effectiveness of INVEGA® Sustenna® in patients < 18 years of age have not been studied. _**Elderly (65 years of age and older)**_ In general, recommended dosing of INVEGA® Sustenna® for elderly patients with normal renal function is the same as for younger adult patients with normal renal function. As elderly patients may have reduced renal function, see _Renal Impairment_ below for dosing recommendations in patients with renal impairment. _**Renal Impairment**_ INVEGA® Sustenna® has not been systematically studied in patients with renal impairment (see _Pharmacokinetic Properties_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). For patients with mild renal impairment (creatinine clearance ≥ 50 to < 80 mL/min), recommended initiation of INVEGA® Sustenna® is with a dose of 100 mg on treatment day 1 and 75 mg one week later, both administered in the deltoid muscle. Thereafter, follow with monthly injections of 50 mg in either the deltoid or gluteal muscle, adjusted within the range of 25 to 100 mg based on patient tolerability and/or efficacy. INVEGA® Sustenna® is not recommended in patients with moderate or severe renal impairment (creatinine clearance < 50 mL/min). _**Hepatic Impairment**_ INVEGA® Sustenna® has not been studied in patients with hepatic impairment. Based on a study with oral paliperidone, no dose adjustment is required in patients with mild or moderate hepatic impairment. Paliperidone has not been studied in patients with severe hepatic impairment (see _Pharmacokinetic Properties_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _**Other Special Populations**_ No dose adjustment for INVEGA® Sustenna® is recommended based on gender, race, or smoking status (for pregnant women and nursing mothers, see _Pregnancy and Breast-feeding_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Switching From Other Antipsychotic Agents** There are no systematically collected data to specifically address switching patients with schizophrenia or schizoaffective disorder from other antipsychotics to INVEGA® Sustenna®, or concerning concomitant administration with other antipsychotics. For patients who have never taken oral paliperidone or oral or injectable risperidone, tolerability should be established with oral paliperidone or oral risperidone prior to initiating treatment with INVEGA® Sustenna® (see _Dosage and Administration_). Previous oral antipsychotics can be immediately or gradually discontinued at the time of initiation of treatment with INVEGA® Sustenna®. INVEGA® Sustenna® should be initiated as described at the beginning of _Dosage and Administration_ above. Patients previously stabilized on different doses of paliperidone extended-release tablets can attain similar paliperidone steady-state exposure during maintenance treatment with INVEGA® Sustenna® monthly doses as depicted in Table 1. 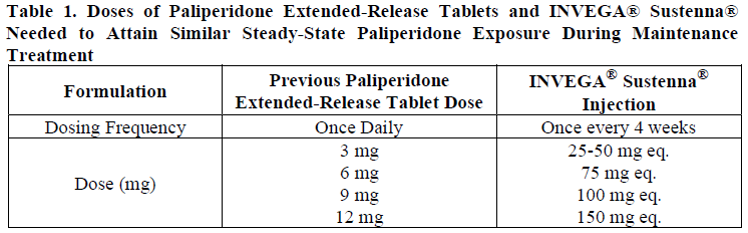 When switching patients currently at steady-state on a long-acting injectable antipsychotic, initiate INVEGA® Sustenna® therapy in place of the next scheduled injection. INVEGA® Sustenna® should then be continued at monthly intervals. The one-week initiation dosing regimen as described at the beginning of _Dosage and Administration_ above is not required. Patients previously stabilized on different doses of risperidone injection can attain similar active moiety steady-state exposure during maintenance treatment with INVEGA® Sustenna® monthly doses as depicted in Table 2. 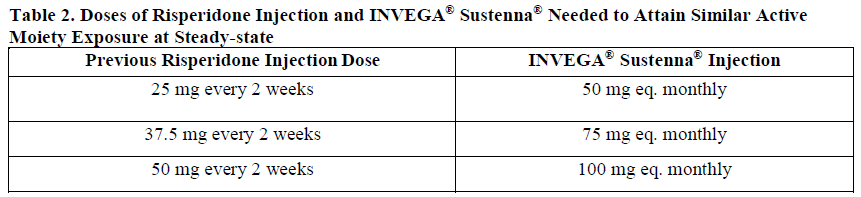 Discontinuation of the previous antipsychotic should be made in accordance with the appropriate prescribing information. If INVEGA® Sustenna® is discontinued, its prolonged-release characteristics must be considered. As recommended with other antipsychotic medications, the need for continuing existing extrapyramidal symptoms (EPS) medication should be re-evaluated periodically.
INTRAMUSCULAR
Medical Information
**Indications** INVEGA® Sustenna® is indicated for the treatment of schizophrenia and for the prevention of recurrence of symptoms of schizophrenia. INVEGA® Sustenna® is indicated for the treatment of schizoaffective disorder as monotherapy and as an adjunct to mood stabilizers or antidepressants.
**Contraindications** INVEGA® Sustenna® is contraindicated in patients with a known hypersensitivity to paliperidone or to any of the components in the formulation. Since paliperidone is an active metabolite of risperidone, INVEGA® Sustenna® is contraindicated in patients with a known hypersensitivity to risperidone.
N05AX13
paliperidone
Manufacturer Information
JOHNSON & JOHNSON INTERNATIONAL (SINGAPORE) PTE. LTD.
Janssen Pharmaceutica N.V.,
Cilag AG
Active Ingredients
Documents
Package Inserts
Invega Sustenna_PI.pdf
Approved: March 8, 2023
