Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
CAPSULE
**4.2. Posology and method of administration** The recommended dose of Ibrance is a 125 mg capsule taken orally once daily for 21 consecutive days followed by 7 days off treatment (Schedule 3/1) to comprise a complete cycle of 28 days. When co-administered with palbociclib, the aromatase inhibitor should be administered according to the dose reported in the approved prescribing information. When co-administered with palbociclib, the recommended dose of fulvestrant is 500 mg administered intramuscularly on Days 1, 15, 29, and once monthly thereafter. Please refer to the full prescribing information of fulvestrant. Ibrance should be taken with food. Patients should be encouraged to take their dose at approximately the same time each day. Continue the treatment as long as the patient is deriving clinical benefit from therapy. If the patient vomits or misses a dose, an additional dose should not be taken. The next prescribed dose should be taken at the usual time. Ibrance capsules should be swallowed whole (do not chew, crush, or open them prior to swallowing). No capsule should be ingested if it is broken, cracked, or otherwise not intact. Prior to the start of, and throughout treatment, pre/perimenopausal women treated with the combination Ibrance plus aromatase inhibitor/fulvestrant should also be treated with luteinizing hormone-releasing hormone (LHRH) agonists according to local clinical practice. For men treated with combination Ibrance plus aromatase inhibitor therapy, consider treatment with an LHRH agonist according to current clinical practice standards. **Dose modifications** Dose modification of Ibrance is recommended based on individual safety and tolerability. Management of some adverse reactions may require temporary dosing interruptions/cycle delays, and/or dose reductions, or permanent discontinuation as per dose reduction schedules provided in Tables 1, 2, and 3 (see Section 4.4 Special warnings and precautions for use and Section 4.8 Undesirable effects – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). 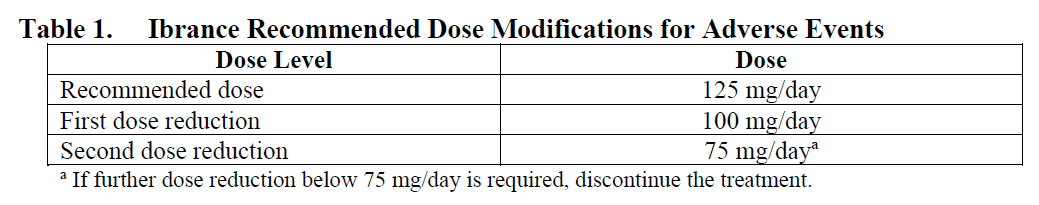 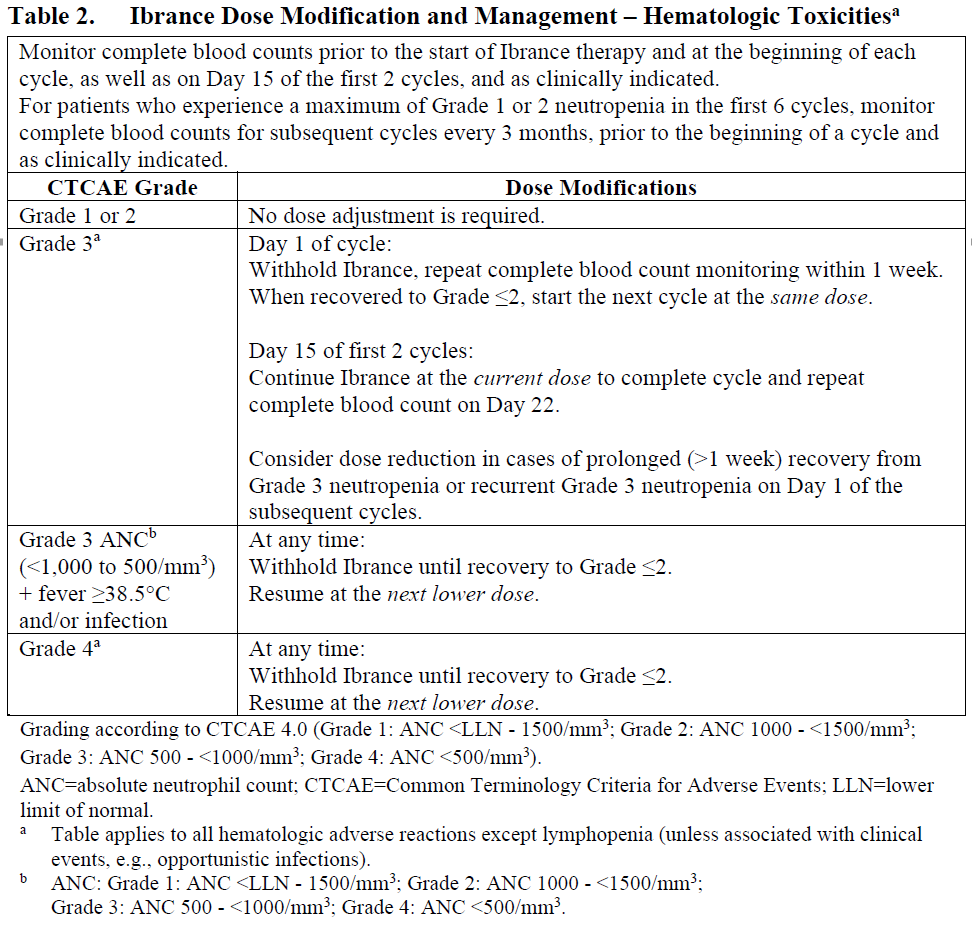 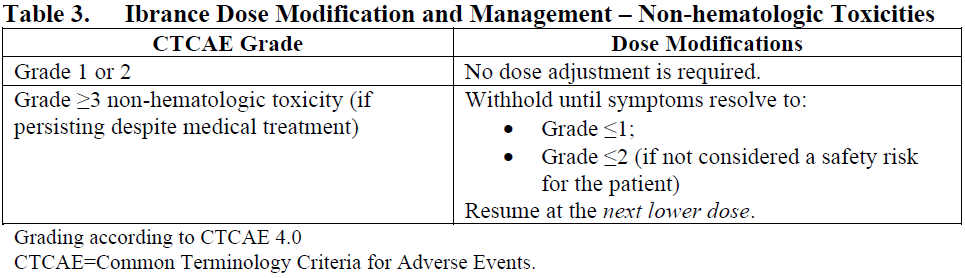 No dose modifications are required on the basis of patient’s age, sex or body weight (see Section 5.2 Pharmacokinetic properties – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Permanently discontinue Ibrance in patients with severe interstitial lung disease (ILD) or pneumonitis (see Section 4.4 Special warnings and precautions for use – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Special populations** _Elderly population:_ No dose adjustment is necessary in patients ≥65 years of age (see Section 5.2 Pharmacokinetic properties – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Pediatric population:_ The safety and efficacy of Ibrance in children and adolescents <18 years of age have not been established. _Hepatic impairment:_ No dose adjustment is required for patients with mild or moderate hepatic impairment (Child-Pugh classes A and B). For patients with severe hepatic impairment (Child-Pugh class C), the recommended dose of Ibrance is 75 mg once daily on Schedule 3/1 (see Section 5.2 Pharmacokinetic properties – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Renal impairment:_ No dose adjustment is required for patients with mild, moderate or severe renal impairment (creatinine clearance \[CrCl\] ≥15 mL/min). Insufficient data are available in patients requiring hemodialysis to provide any dosing recommendation in this patient population (see Section 5.2 Pharmacokinetic properties – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
ORAL
Medical Information
**4.1. Therapeutic indications** Ibrance is indicated for the treatment of patients with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced or metastatic breast cancer in combination with: - an aromatase inhibitor as initial endocrine-based therapy; or - fulvestrant in patients with disease progression following endocrine therapy.
**4.3. Contraindications** None
L01XE33
xl 01 xe 33
Manufacturer Information
PFIZER PRIVATE LIMITED
Pfizer Manufacturing Deutschland GmbH
