Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
CREAM
**Dosage & application** Posology To achieve a lasting cure, treatment with bifonazole must be carried out reliably and over an adequate period. The usual periods of treatment are summarized in the table below: 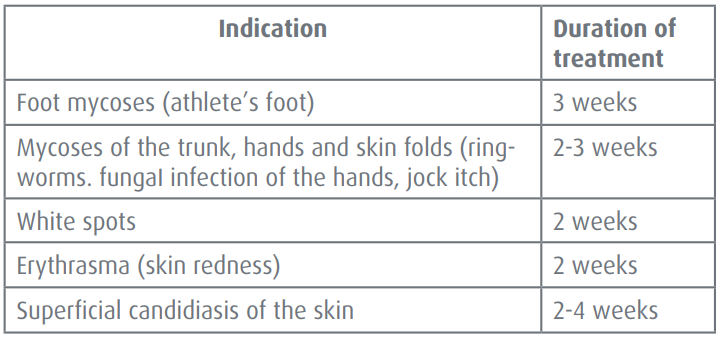 **Method of administration** Once a day, preferably in the evening, before going to bed. It should be applied thinly to the affected skin area and rubbed in. A small amount of cream is generally sufficient to treat an area of about the size of the palm of hand. **Use in Children** No in-depth studies have been performed in the pediatric population (0 to 18 years). From a survey of the clinical data reported there is no indication that harmful effects should be anticipated in pediatric population. However, in neonates (0 to 27 days), infants and toddlers (28 days to 23 months), the medicinal product should only be used under medical supervision.
TOPICAL
Medical Information
**Indications** - Treatment of skin mycoses caused by dermatophytes, yeasts, moulds, and other fungi (e.g. athlete's foot, fungal infection of the hand, ringworms, jock itch, white spots, superficial fungal skin infections & other tinea conditions). - Treatment of erythrasma (skin redness).
**Contraindication** Hypersensitivity to the active substance or to any of the excipients.
D01AC10
bifonazole
Manufacturer Information
BAYER (SOUTH EAST ASIA) PTE LTD
GP Grenzach Produktions GmbH
Kern Pharma, S.L.
Active Ingredients
Documents
Patient Information Leaflets
Canespro Once Daily Cream 1% - PIL.pdf
Approved: July 29, 2022
