Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
CAPSULE
**DOSAGE AND ADMINISTRATION** The standard doses in Table 1 below are the recommended initial dose for adults according to body surface area. TS-ONE® should be administered twice daily, after breakfast and after the evening meal, for 28 consecutive days, followed by a 14-day rest. This is regarded as one course of the regimen. 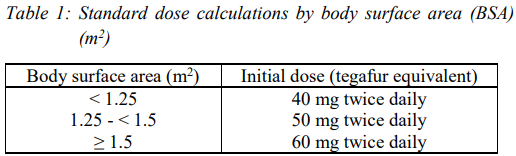 The initial dose can be decreased according to the patient's tolerance to the medication. The reduction of dose may be done in 10 mg intervals, with a lower limit of 40 mg. **Precautions on Dosage and Administration** 1. When the dose is decreased according to the patient's condition, the following standard doses should be referenced. 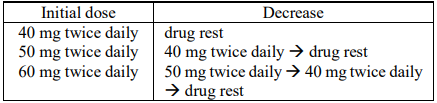 2. If a drug rest period therapeutically needs to be shortened, it should be implemented after confirming that no drug-induced abnormalities in laboratory findings (hematological tests, liver and renal function tests) and no gastrointestinal symptoms occur, i.e., the drug is not problematic in terms of safety. A minimum drug rest period of 7 days must be provided. 3. To avoid serious adverse reactions such as bone marrow depression and fulminant hepatitis, the patient's condition should be monitored thoroughly by performing laboratory tests (hematological tests, liver and renal function tests) before the start of each course and at least once every 2 weeks during dosing. If any abnormal findings are observed, appropriate measures should be taken, such as prolongation of the drug rest period, dosage reduction according to the above-mentioned standard doses, or discontinuing administration of TS-ONE®. 4. Since basic investigations (rats) have revealed that the bioavailability of oteracil potassium changes when the drug is administered in the fasting state, it is speculated that phosphorylation of fluorouracil is inhibited and that its antitumor effect is reduced. TS-ONE® should be administered after meals. 5. The recommended treatment course for post-operative adjuvant chemotherapy for gastric cancer is one year after surgery. Treatment with TS-ONE® beyond one year after surgery has not been studied.
ORAL
Medical Information
**INDICATIONS** - Post-operative adjuvant chemotherapy for locally advanced (stage II (excluding T1), IIIA or IIIB) gastric cancer - For the treatment of locally advanced or metastatic adenocarcinoma of the pancreas - For the treatment of locally advanced or metastatic non-small cell lung cancer in patients who have been previously treated with platinum-based chemotherapy
**CONTRAINDICATIONS** **(TS-ONE® is contraindicated in the following patients.)** 1. Patients with a history of severe hypersensitivity to the ingredients of TS-ONE® 2. Patients with severe bone marrow depression \[Bone marrow depression may be aggravated.\] 3. Patients with severe renal disorder \[The urinary excretion of gimeracil, a catabolic enzyme inhibitor of fluorouracil (5-FU), is markedly decreased, thereby the blood concentration of 5-FU is increased. These suggest that adverse reactions such as bone marrow depression may be enhanced (See Pharmacokinetics – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).\] 4. Patients with severe hepatic disorder \[Hepatic disorder may be aggravated.\] 5. Patients receiving treatment with other fluoropyrimidine-group anti-cancer drugs including combination therapies with them (See Drug Interactions – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) 6. Patients receiving treatment with flucytosine (See Drug Interactions – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) 7. Pregnant women or women suspected of being pregnant (See Use during Pregnancy, Delivery or Lactation – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_)
L01BC53
tegafur, combinations
Manufacturer Information
TAIHO PHARMA ASIA PACIFIC PTE. LTD.
Taiho Pharmaceutical Co., Ltd. (Tokushima Plant)
Active Ingredients
Documents
Package Inserts
1.4.3 Proposed TS-ONE PI (SG)_Mar 2022_cln_QCed.pdf
Approved: March 30, 2022
