Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, POWDER, LYOPHILIZED, FOR SUSPENSION
**Posology and method of administration** Pamorelin is administered by a single intramuscular injection. Dosing schedule depends on the product strength selected (see Table 1). 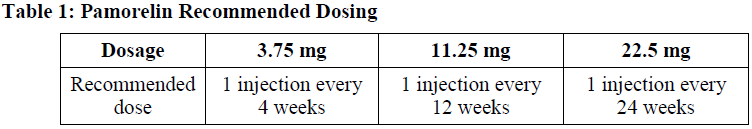 No dosage adjustment is necessary for patients with renal or hepatic impairment. In women the treatment of endometriosis with Pamorelin 3.75 mg begins during the early follicular phase and should not exceed 6 months. Pituitary down-regulation in the context of medically assisted procreation (IVF, GIFT etc.): One intramuscular injection of Pamorelin 3.75 mg administered either in the early follicular phase, usually on the 2nd day of the menstrual cycle, or in the mid-luteal phase, usually on the 21st day of the previous cycle. In general, the stimulation by gonadotrophins should be performed when the plasma levels of estrogens are consistent with ovarian suppression, usually less than 50 pg/ml around the 15th day of the cycle. In high-risk localized or locally advanced hormone-dependent prostate cancer as concomitant to and following radiation therapy, clinical data have shown that radiotherapy followed by long-term androgen deprivation therapy is preferable to radiotherapy followed by short-term androgen deprivation therapy \[see **Clinical efficacy in men with prostate cancer** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_\]. The treatment duration of androgen deprivation therapy recommended by medical guidances is 2–3 years in these patient populations receiving radiotherapy. In patients with metastatic castration resistant prostate cancer not surgically castrated receiving a GnRH agonist, such as triptorelin, and eligible for treatment with an inhibitor of androgen biosynthesis (e.g. abiraterone acetate), or an inhibitor of androgen receptor function (e.g. enzalutamide), treatment with the GnRH agonist should be continued. The treatment of children with Pamorelin 22.5 mg should be under the overall supervision of a paediatric endocrinologist or of a paediatrician or an endocrinologist with expertise in the treatment of central precocious puberty. Treatment should be stopped around the physiological age of puberty in boys and girls and should not be continued in girls with a bone maturation of more than 12–13 years. There are limited data available in boys relating to the optimum time to stop treatment based on bone age, however it is advised that treatment is stopped in boys with a bone maturation age of 13–14 years. Method of Administration The lyophilised microgranules are to be reconstituted using 2 mL of sterile water for injection (see **Special precautions for disposal and other handling** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The solvent for suspension should be drawn into the injection syringe and transferred to the vial containing the powder. The vial should be agitated to thoroughly disperse particles and obtain a uniform suspension. The agitation should be done by moving back and forth from bottom to top and back and forth from left to right alternately. The suspension will appear milky. The suspension obtained should be drawn back into the injection syringe. The injection needle has to be changed and the resulting suspension for injection should be administered immediately. The suspension should be discarded if not used immediately after reconstitution. Once reconstituted, the suspension of Pamorelin should be injected relatively rapidly and in an uninterrupted manner in order to avoid any potential blockage of the needle. Since Pamorelin is a suspension of microgranules for intramuscular (IM) injection only, inadvertent intravascular injection must be strictly avoided. As with other medicinal products administered by injection, the injection site should be varied periodically. Pamorelin must be administered under the supervision of a physician.
INTRAMUSCULAR
Medical Information
**Therapeutic indications** Pamorelin 3.75 mg, 11.25 mg, and 22.5 mg is indicated for the treatment of locally advanced or metastatic, hormone-dependent prostate cancer. Pamorelin 3.75 mg, 11.25 mg, and 22.5 mg are indicated as concomitant to and following radiotherapy in patients with high-risk localized or locally advanced prostate cancer. Pamorelin 3.75 mg is indicated for the treatment of endometriosis. Pamorelin 3.75 mg is indicated for the pituitary down-regulation in the context of assisted reproduction technology. Pamorelin 22.5 mg is indicated for the treatment of central precocious puberty (CPP) in children of 2 years of age and older with an onset of CPP before 8 years in girls and 10 years in boys.
**Contraindications** Pamorelin is contraindicated in patients with known hypersensitivity to triptorelin, GnRH (Gonadotropin releasing hormone), other GnRH agonist analogues or to any of the excipients of Pamorelin. Pamorelin is contraindicated in patients with spinal cord compression secondary to prostate cancer metastases. Pamorelin is contraindicated during pregnancy and lactation period. Pamorelin is contraindicated in patients with unexplained vaginal bleedings.
L02AE04
triptorelin
Manufacturer Information
ORIENT EUROPHARMA PTE LTD
Debiopharm Research & Manufacturing SA
Synergy Health Daniken AG (Contract Sterilization Facility)
Active Ingredients
Documents
Package Inserts
Pamorelin Package Insert.pdf
Approved: September 6, 2021
